Remember Me


Experiment Transpiration of Plants Experiments
Transpiration of plants.
Experiment #13 from Investigating Biology through Inquiry
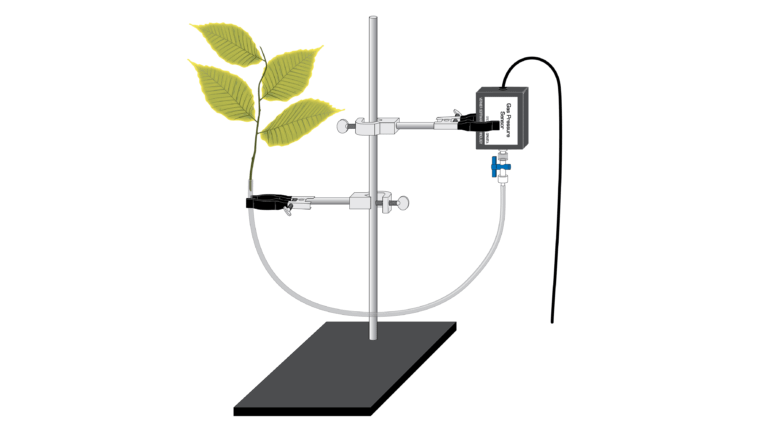
Introduction
In plants, water is transported from the roots to the leaves, following a decreasing water potential gradient. Transpiration , or loss of water from the leaves, helps to create a lower osmotic potential in the leaf. The resulting transpirational pull is responsible for the movement of water from the xylem to the mesophyll cells into the air spaces in the leaves. The rate of evaporation of water from the leaf to the outside air depends on the water potential gradient between the leaf and the outside air. Various environmental factors, including those conditions which directly influence the opening and closing of the stomata, will also affect a plant’s transpiration rate.
In this Preliminary Activity, you will use a Gas Pressure Sensor to measure transpiration rates under different conditions. The data will be collected by measuring pressure changes as the plant takes up water into the stem.
After completing the Preliminary Activity, you will first use reference sources to find out more about transpiration before you choose and investigate a researchable question dealing with transpiration rate. Some topics to consider in your reference search are:
- transpiration
- cohesion-tension theory
- water potential
- water potential gradient
- mesophyll cell
Sensors and Equipment
This experiment features the following sensors and equipment. Additional equipment may be required.

Correlations
Teaching to an educational standard? This experiment supports the standards below.
Ready to Experiment?
Ask an expert.
Get answers to your questions about how to teach this experiment with our support team.
- Call toll-free: 888-837-6437
- Chat with Us
- Email [email protected]
Purchase the Lab Book
This experiment is #13 of Investigating Biology through Inquiry . The experiment in the book includes student instructions as well as instructor information for set up, helpful hints, and sample graphs and data.


BIOLOGY JUNCTION
Test And Quizzes for Biology, Pre-AP, Or AP Biology For Teachers And Students
Lab 9 Transpiration Example 2 ap
Introduction
Most of the water a plant absorbs is not used for a plant’s daily functioning. It is instead lost through transpiration, the evaporation of water through the leaf surface and stomata, and through guttation, which is the loss of water from the vascular tissues in the margins of leaves.
There are three levels of transport in plants: uptake and release of water and solutes by individual cells, short distance cell to cell transport at tissue and organ levels, and long distance transport of sap by xylem and phloem at the whole plant level. The transport of water is controlled by water potential. Water will always move from an area of high water potential to an area with low water potential. This water potential is affected by pressure, gravity, and solute concentration.
Water moves into the plant through osmosis and creates a hydrostatic root pressure that forces the water upward for a short distance, however, the main force in moving water is the upward pull due to transpiration. This pull is increased by water’s natural properties such as adhesion and cohesion. Transpiration decreases the water potential in the stele causing water to move in and pull upward into the leaves and other areas of low water potential. Pressure begins to build in the leaves, so to prevent downward movement, guttation occurs. Guttation occurs through leaf openings on the leaf margins called hydrathodes. Loss of water through transpiration can be facilitated by the opening and closing of the stomata depending on environmental conditions.
There are three types of cells in plants: parenchyma, sclerenchyma, and collenchyma. Parenchyma cells are the most abundant and are not specialized. They are found in the mesophyll of leaves, the flesh of fruits, the pith of stems, and the root and stem cortex. Sclerenchyma are elongated cells that make up fibers. They have thick secondary walls and the protoplasts often die as they grow older. They are used for support and are found in vascular tissue. Collenchyma cells are living at maturity and have a thickened secondary wall.
In Lab 9A, all of the plants in this experiment will lose water through transpiration, but those affected by the heat sink and the fan will lose a larger amount of water due to the environmental conditions. This transpiration will pull water from the potometer into the plant. The structure and cell types of a stem cross-section can be observed under a microscope.
Exercise 9A: Transpiration
The materials needed for this exercise were a pan of water, timer, a beaker containing water (heat sink), scissors, 1-mL pipette, a plant cutting, ring stand, clamps, clear plastic tubing, petroleum jelly, a fan, lamp, spray bottle, a scale, calculator, and a plastic bag.
Exercise 9B: Structure of the Stem
The materials needed for this exercise were a nut-and-bolt microtome, single-edge razor blade, plant stems, paraffin, 50% ethanol, distilled water, 50% glycerin, toluidine blue O stain, a microscope slide and cover slip, pencil, paper, and a light microscope.
The tip of the pipette was placed in the plastic tubing and they were submerged in a tray of water. Water was drawn into the pipette and tubing until no bubbles were left. The plant stem was cut underwater and inserted into the plastic tubing. Petroleum jelly was immediately placed around the tube edging to form an airtight seal around the stem. The tubing was bent into a “U” shape and two clamps were used on the ring stand to hold the potometer in place. The potometer was allowed to equilibrate for ten minutes.
The plant was exposed to a fan, which was placed one meter away and set on low speed. The time zero reading was recorded and then it was continually recorded every three minutes for 30 minutes. After the experiment, all the leaves were cut off the plant and massed by cutting a one cm2 box and massing it.
A nut-and-bolt microtome was obtained and a small cup was formed by unscrewing the bolt. The stem was placed in the microtome and melted paraffin was poured around the stem. The paraffin was allowed to dry and the excess stem was cut off. The bolt was twisted just a little and then cut with the blade. The slice was placed in the 50% ethanol. The slices were left in the ethanol for five minutes. Using the forceps, the slices were moved to a dish of the toluidine blue O stain and left for one minute. The sections were rinsed in distilled water. The section was mounted on the slide with a drop of 50% glycerin. A cover slip was placed over the slide. The cross section was observed under a light microscope and drawn.
Table 9.1: Individual Potometer Readings
Class Potometer Readings
Mass of leaves = 1.1 g Leaf Surface Area = 0.0044 m 2
Table 9.2: Individual Water Loss in mL/m2
Table 9.3: Class Average Cumulative Water Loss in mL/m2
Analysis of Results
Calculate the average rate of water loss per minute for each of the treatments: Room: 1.67 mL/m2 Fan: 0.76 mL/m2 Light: 0.93 mL/m2 Mist: 0.83 mL/m2
Explain why each of the conditions cause an increase or decrease in transpiration compared with the control.
How did each condition affect the gradient of the water potential from stem to leaf in the experimental plant?
The light and the fan decreased the water potential in the leaves and water moved up the stem by transpiration pull. The room temperature had little or no effect on the water potential. The mist increased the water potential of the air causing less transpiration to occur from the leaves.
What is the advantage to a plant of closed stomata where water is in short supply? What are the disadvantages?
The closing of the stomata would prevent transpiration of water and minimize this loss if water was in short supply. It is a conservational adaptation. However, closing stomata prevents the exchange of gases in plants and limits their carbon supplies.
Describe several adaptations that enable plants to reduce water loss from their leaves. Include both structural and psychological adaptations.
Plants that are adapted to drier climates are called xerophytes. Some of these plants have adapted small, thick leaves with a reduced surface area. They may also have a thickened cuticle to protect themselves from the environment. The stomata may be sunken into pits. Some xerophytes shed their leaves during the driest seasons and others can store water such as cacti. CAM plants uptake CO2 at night and change it into crassulacean acid that can be broken down during the day for sugars. These plants can close their stomata during the day.
Why did you need to calculate leaf surface area in tabulating your results?
The surface area has to be calculated because this greatly affects the amount of water lost through transpiration. Smaller leaves may lose less water than the larger ones, but by calculated water loss by surface area creates comparable data that is constant and consistent.
Error Analysis
This lab had many opportunities for error. The potometer set up was a complicated procedure. If any air bubbles were present in the plastic tubing, it could cause drastic error to occur. Any miscalculations or inaccurate weighing could also account for error.
Discussion and Conclusion
Transpiration in plants is controlled by water potential. This change in water potential in leaves causes a gradient by which water can be moved upward. When the water potential of the air was increased by the mist and plastic bag, less water evaporated from the leaves, decreasing the water potential gradient between the root and stem. This decreased the transpiration pull. The fan and floodlight simulated environmental conditions such as wind, heat, and intense light. These conditions increase the amount of water transpired by plants. This in turn increased the water potential gradient causing more water to be pulled through the stem. The control plant should have had normal rates of transpiration.
The stem must have specialized cells for support and transport. The epidermis is the outermost layer of the stem. The xylem is a transport tube for water, and the phloem transports food and minerals through the plant. Parenchyma are non-specialized cells and are located in the interior. The tougher sclerenchyma and collenchyma make up the structural outer support of the epidermis and the transport tubes of phloem and xylem.
- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now

Experiments on Transpiration in Plants | Botany
ADVERTISEMENTS:
List of top nine experiments on transpiration in plants:- 1. Measurement of Leaf Area 2. Demonstration of Transpirational Water Loss by Potometers 3. Determination of the Rate of Transpiration by Simple Method (Conical Flask Method) 4 . Determination of the Rates of Stomatal and Cuticular Transpiration and a few others.
Experiment # 1. Measurement of Leaf Area :
The loss of water in the form of vapour from the aerial parts — particularly through leaves — is termed “transpiration”. On absorption from the soil by roots, the water is trans-located via the xylem tissue to the mesophyll cells of the leaves.
The excess water is lost through stomatal opening or through the diffusion process from leaf surface. For determination of the rate of transpiration, measurement of leaf area, i.e. transpiring surface, is highly essential. The leaf area can be measured by different methods.
Method I: By Graph Paper Method:
Requirements :
1. Graph paper (mm); 2. Scale, pencil, leaf, etc.
Procedure :
1. Place leaf on a millimeter graph paper and draw its outline with a pencil (Fig. 3.11).

2. Then count the total area covered by the leaf from the marked outline of the leaf and express it as square centimeter.
Observation :
The leaf area measurement procedure is shown in Fig. 3.11.
Method II: By Weighing Method:
1. Card-board
2. Leaf, pencil, rubber, blade, balance with weight box.
1. Place a leaf on a cardboard and draw its outline. Then cut the board along the line-mark of drawing and take its weight.
2. Now cut one square centimeter area from the board and take the weight. Observation
The weight of the board cut to the size of leaf area is x gms. The weight of one square centimeter area of the board is y gms. Then the area of leaf is x/y sq. cm (Fig. 3.12).

Method III: By Planimeter Method:
1. Planimeter — a simple instrument, having two major parts — a tracer arm having a tracing point and a carriage with a measuring wheel and also the pole arm attached to the pole, around which the instrument revolves (Fig. 3.13).

2. A platform, leaf, pencil etc.
1. Place the leaf on a platform in a fixed position and draw the outline of the leaf by a pencil.
2. Place the pole weight close to the outline of the leaf and move the tracer point along the margin of the leaf.
3. Record the initial reading from the scale and final reading after the tracing of leaf.
4. Compute the total leaf area by denoting the data from the main scale and also from vernier scale.
5. Record the vernier scale reading in the following ways before final computation:
First coincide the zero of the measuring wheel with zero of the vernier scale. Find out the number of divisions of the vernier scale that coincides with that of the measuring wheel.
Suppose 10 vernier divisions = 9 divisions of measuring wheel. So, 1 vernier division = 9/10 or 0.9 division of the measuring wheel. Vernier unit = 1 – 0.9 = 0.1 sq. cm small division of the measuring wheel = 1 sq. cm and 1 div. of the counter dial = 100 sq. cm.
Thus total reading = (Counter dial reading × 100) + (Measuring wheel reading × 10) + (vernier reading × 0.10) sq. cm.
Experiment # 2. Demonstration of Transpirational Water Loss by Potometers:
The water loss by the process of transpiration can be demonstrated by several types of glass apparatus, called Potometers. In most of the potometers, the rate of transpiration can be measured directly and expressed in gms per hour per sq. cm of leaf area.
But these methods are not accurate because of the fact that the amount of water absorbed by the twig (which is measured by the apparatus) is not actually transpired at the same time.
The description and working of some potometers are given below:
(A) Ganong’s Proto-Meter (Fig. 3.14):

This is a glass apparatus fitted with a wooden stand. It is one of the most suitable potometers used for the demonstration or determination of the rate of transpiration.
It consists of a narrow graduated horizontal limb which holds two vertical wide-mounted tubes — one of which is fitted with a rubber cork through which passes a leafy twig while the other acts as a reservoir of water which is fitted with a stopcock in the connecting tube to control water supply. The other ends of horizontal limbs bend at right angle and at the opposite side of the vertical wide mouthed tube.
Materials Required :
1. Ganong’s potometer
2. Water, beaker, leafy twig, knife, etc.
3. Graph paper, pencil, etc.
1. Fill the apparatus with water and insert a leafy twig (cut under water) through the cork of the vertical tube. The twig should always be cut under water to prevent air-clogging.
2. Keep some water in the reservoir funnel and close the stopcock and make all the connections air-tight by proper sealing.
3. Introduce a drop of air bubble in the horizontal limits of the apparatus.
4. Allow the twig to transpire for 1-2 hrs. under bright sunshine.
As water is lost by transpiration, the bubble will move in the horizontal graduated tube towards the transpiring twig. The rate of movement of the bubble in the horizontal tube is proportional to transpiration rate (assuming that the rates of absorption and transpiration are the same).
The rate of transpiration can be determined in the following ways:
Initial position of the bubble on the scale — X cm
Final position of the bubble after a given time — Y cm
Therefore, the distance traversed by the bubble in time t is equal to (Y – X) cm.
Now the volume of water transpired in a given time (t) is equal to tit 2 (Y – X) ml where ‘r’ is the radius of the bore of the horizontal tube.
So, the amount of water transpired by per unit area of the leaves of the twig per unit time is equal to
(Y – X)/ t x total leaf area * ml/min/sq. cm.
[* The leaf area can be measured by graph paper method.]
(B) Farmer’s Potometer (Fig. 3.15):

The apparatus consists of a wide-mouthed bottle fitted with a rubber stopper having three holes. The bottle is filled with water up to the neck. In one hole leafy twig can be introduced while in another a water reservoir having a stopcock is fitted. The third hole is fitted with a narrow bent tube which has a horizontal graduated tube with a centimeter scale.
1. Farmer’s potometer
3. Water, leafy twig, pencil, graph paper etc.
1. Fill the apparatus with water and keep some water in the reservoir.
2. Introduce a freshly cut (cut under water) twig within the bottle and make all connections air-tight by proper sealing.
3. The bent end of the narrow tube is to be immersed in a beaker containing water.
4. Keep the whole set-up under bright sunshine for transpiration at a steady state.
Observe the movement of the air-bubble within the horizontal tube towards the twig. The rate of movement of air-bubble is proportional to the rate of transpiration (assuming that the rates of absorption and transpiration are equal).
Same as in Ganong’s potometer.
(C) Darwin’s Potometer (Fig. 3.16):

The apparatus consists of a short glass tube from which a side tube bends upward ending in an open mouth into which a plant twig is inserted through a hole in a rubber cork.
The upper open mouth of the main tube is also closed by a cork. The lower end of the tube too is fitted with a cork through which passes a long graduated capillary tube, fitted with the help of a rubber tubing. The end of the capillary tube dip in a beaker containing.
1. Darwin’s potometer
2. Beaker, leafy twig, water, graph paper, pencil etc.
1. At the beginning of the experiment, fill up the apparatus with water.
2. Insert a fresh leafy twig (cut under water) through the cork of the side tube.
3. Make all joints air-tight.
4. Introduce an air-bubble within the water column of the capillary tube.
5. Allow the whole set to transpire under bright light after fixing it with stand and clamp.
As transpiration occurs from leaves of the twig water is absorbed by the twig from the side tube and this produces a suction force which sucks up water from the capillary tube. As a result, the air-bubble within the capillary tube gradually moves upward.
The rate of upward movement of air bubble is recorded from the initial and final readings of the position of the air-bubble in the capillary tube. The rate of transpiration is then expressed as in case of Ganong’s Potometer (ml of water transpired per minute per unit area of the leaf).
(D) By Garreau’s Potometer (Fig. 3.17):

It consists of two small bell jars placed one above another in-between which a leaf is placed while still attached to a potted plant. At the narrow end of the two bell jars, weighed amounts of anhydrous CaCl 2 are placed in two very small tubes. At the two ends of the bell jars, are attached two oil manometers which ensure the maintenance of constant vapour pressure within the bell jars.
Materials and Equipment’s :
1. Garreau’s Potometer
2. Vaseline, Anhydrous CaCl 2 salt
3. Potted plant, stand with clamps
4. Balance with weight box
1. Place a leaf of a potted plant inside two bell jars and make it airtight by vase-line.
2. Clamp the whole arrangement of the apparatus in vertical position and place it in sunlight.
3. Before the onset of the experiment place measured quantities of anhydrous CaCl 2 salt in the tubes and take the final weight after a considerable period of transpiration (at least two hours).
The difference between the two weighing’s is a measure of the amount of water lost from the upper and lower leaf surfaces.
Hence transpiration by both the surface of a leaf can be directly measured separately and simultaneously by Garreau’s potometer:
Initial weight of CaCl 2 in the upper tube — W 1 gms
Initial weight of CaCl 2 in the lower tube — W 2 gms
Final weight of CaCl 2 in the upper tube — W 3 gms
Final weight of CaCl 2 in the lower tube — W 4 gms
Amount of transpired water by upper leaf surface — (W 3 – W 1 ) gm.
Amount of transpired water by lower leaf surface — (W 4 – W 2 )
Rate of upper leaf surface transpiration i.e. cuticular transpiration (in case of dorsiventral leaf) = (W 3 – W 1 ) gm. total leaf area (sq m) and time (min)
Rate of lower leaf surface transpiration i.e. stomatal transpiration = (W 4 – W 2 ) gm. total leaf are (sq m) time (min)

Experiment # 3. Determination of the Rate of Transpiration by Simple Method (Conical Flask Method) :
The loss of water from the leaf surface of terrestrial plants is a normal physiological process. It is either transpirational loss, i.e., in the form of water vapour, or guttation i.e., in the form of water droplets.
Transpiration normally takes place through stomatal openings of leaves or through particular openings of stem surface or through the cuticular surface, or a combination of paths mentioned above. This transpirational water loss is a necessary evil for plant life.
Transpirational water loss can be determined by the conical flask method — a very simple method.
Materials and Equipments :
1. A fresh leafy twig
2. Beaker, conical flask, knife, glass rod, thread etc.
3. Water, oil, balance with weight box
4. Graph paper, pencil, stop-watch etc.

1. Take a 100 ml conical flask and fill it with water up to the neck.
2. Insert a freshly cut petiolate leaf (leaf cut under water within a breaker) and tie the cut end of the petiole with a glass rod by thread so that the leaf cannot be displaced from the conical flask by wind.
3. Then put some oil over the water of conical flask so that the exposed water surface will be covered.
4. Weigh the experimental set (conical flask – water-oil-leaf) in a chemical balance and record the initial weight.
5. Place the experimental set under bright sunshine for 1 hour and weight finally in a chemical balance.
6. Record the final weight and calculate the water loss by transpiration.
7. Record the total transpiring area of the leaf by Graph paper method.
8. Determine the rate of transpiration in the following way:

Stomatal Index (S.I.) = No. of stomata in a given area (S)/Total no. of cells of the area (epidermal) + S × 100
6. Determine the total area of the leaf by graph paper method and then calculate the total number of stomata of the said leaf.
7. Determine the area covered by each stomata with the help of ocular micrometer (value of each division is standardized before by the stage micrometer by the formula):
1 ocular division value = Stage div./Ocular div. × 10µ.
Then calculate the total stomatal area of the given leaf.
8. Determine the transpiration index by recording the time (in sec) required for standard change of the dry cobalt chloride paper over the evaporating surface (S) and transpiring surface (E) by using the formula:
Transpiration index = S/E × 100
In this process, take two equal pieces (2 × 2 cm) of dry cobalt chloride paper and then place one of them under the lower surface of dorsiventral leaf of a twig by cello tape. Place the other over a wire net which is kept over a petridish containing water. Record the time (in sec) for a standard colour change of the cobalt chloride papers in both the cases (the paper turns pink when it absorbs water vapour moisture).
Area of the field of vision = x sq cm (calculate it by the formula πr 2 , where ‘r’ is the radius of the field)
Number of stomata per field = a
Total leaf area (from graph paper) = y sq cm
Stomatal frequency = a/x
Total number of stomata of the leaf = a/x × y

Stomatal index = No. of stomata in a given area(S)/Total no. of epidermal cell (E) + S = S/E + S x 100
Area of a stomata = π/4 (b x c) sq cm
Where ‘b’ and ‘c’ represent the length and the breadth of the pore.
Total stomatal area of leaf = π/4(b x c) x (a/x × y) sq cm
[S = Time taken to change the colour of cobalt chloride paper from a free evaporating surface; E = Time taken to change the colour of cobalt chloride paper from a free transpiring surface.]
Experiment # 6 . Determination of the Amount of Water Absorbed and Transpired by a Plant:
Absorption of water and subsequent loss of it in the form of vapour by the aerial parts of plants are two essential interlinked physiological processes. There is positive correlation between the two processes.
In normal situation the amount of water absorbed is much higher than the amount of water transpired. But under stressed conditions the amount of water transpired may be higher than the amount of water absorbed.
The relationship between the two processes mentioned above can be determined by two experimental procedures:
(a) Direct determination with the help of glass apparatus.
(b) By conical flask-water-oil-leaf experimental sets.
(a) Direct Measurement Method:
Materials and Equipment’s:
1. Glass apparatus:
This is a simple apparatus consisting of a wide-mouthed bottle with a graduated side tube (in ml) attached to its base through a cork. The mouth of the bottle is fitted with a cork through which a small plant can be introduced (Fig. 3.19).

2. A small rooted plant or a fresh twig
3. Oil, water, sealing wax, etc.
1. Fill the apparatus completely with water.
2. Introduce a rooted plant or a fresh twig (cut under water) in the wide-mouthed bottle through the cork).
3. Seal the cork to make air-tight.
4. Put a few drops of oil on the surface of water of the graduated side tube to check surface evaporation.
5. Record the initial weight and initial water level on the side tube.
6. Keep the whole set under sunshine for 2 hours.
7. Record the final weight and the level of water in the side tube.
8. Calculate the amount of water transpired (in gms). (Initial weight – final weight) and the amount of water absorbed (in ml) (initial reading – final reading of the side tube).
The volume of water absorbed may be converted to gm. by multiplying the density of water at that temperature from a standard temperature density table.
Initial weight of the experimental set — W 1 gms
Final weight of the experimental set — W 2 gms
Thus the amount of water transpired = (W 1 – W 2 ) gm. = x gm.
Initial reading of side tube = 0 ml
Final reading of side tube = p ml
Thus the amount of water absorbed = (o – p) ml = Q ml
If the density of water is d, so Q ml of water = Q × d gm. = y gms
So the ratio of water transpired and water absorbed is x: y.
(b) By Conical Flask-Water-Oil-Leaf Method (Fig. 3.18) :
Materials and Equipment’s Required :
1. Conical flask, glass rod, thread etc.
2. Water, oil, leafy twig etc.
3. Balance with weight box
1. Take a conical flask (100 ml), fill it up to the neck with water.
2. Put a very little amount of oil over the water surface and take the initial weight (W 1 ) gms.
3. Slowly incline the flask and insert a fresh petiolate leaf (cut under water) tied to a short glass rod with a thread. Care must be taken that oil does not stick to the cut surface of the leaf.
4. Take the second weight (W 2 gms) of the set (conical flask – water-oil-leaf).
5. Place the experimental set under sunshine for 1 hour for transpiration and then take the final weight of the set with leaf – (W 3 gms) and without leaf – (W 4 gms).
6. Now calculate the amount of water transpired and amount of water absorbed.
Amount of water absorbed = (W 1 – W 4 ) gms
Amount of water transpired = (W 2 – W 3 ) gms
The difference between the two values gives the amount of water retained by the leaf or the excess water transpired — as the case may be.
Experiment # 7 . Compare the Rate of Transpiration With the Rate of Evaporation :
The process of vaporization of water from the exposed surface of water and that from the leaf surface are called evaporation and transpiration, respectively. The former is a physical while the latter is a physiological process.
The rate of evaporation is dependent on environmental factors like temperature, humidity, wind velocity, etc. while the rate of transpiration is dependent on both environmental and plant factors, particularly the water retention capacity of the plant concerned.
1. Conical flask, petridish, balance with weight box, glass rod, thread, etc.
2. A leafy twig
3. Water, oils, graph paper, pencil, etc.
1. Prepare a transpirational experimental set (conical flask-water-oil-leaf) in the usual way.
2. Take a petridish and fill it with water up to 2/3 of its volume. This is the evaporation experimental set.
3. Take the initial weight of the sets.
4. Both the sets are placed under sunshine for 1 hour.
5. Take the final weight of the sets and measure the area of transpiration surface and evaporation surface.
6. Calculate the rate of transpiration per min per sq cm of leaf area, and also the rate of evaporation per min per sq cm.
(a) Transpiration set:
Initial weight — W 1 gm.
Final weight — W 2 gms
Amount of water transpired = (W 1 – W 2 ) gm. = x gm.
Total transpiring surface (from graph paper) = y sq cm
Time — 1 hour
Rate of transpiration = x/y × 60 gm. per min per sq cm
(b) Evaporation set:
Initial weight — W 3 gms
Final weight — W 4 gms
Amount of water evaporated = (W 3 – W 4 ) = p gm.
Total evaporating surface (from graph paper) = Q sq cm
(Apply the formula Hr 2 to find out the total evaporating surface)
Time — 1 hour.
Rate of Evaporation = P/Q × 60 gms per min per sq cm
Ratio of Transpiration and Evaporation = X/60Y: P/60Q.
Experiment # 8 . Determination of the Effect of Antitranspirant Chemical on Transpiration :
The term “antitranspirant” is used to designate any material applied to plants for the purpose of retarding transpiration. There are different groups of antitranspirant chemicals — some of them simply act as permeability barrier, some may act as metabolic inhibitors, while some may also act through permeability changes of the guard cells.
Phenyl mercuric acetate is one of the potent antitranspirant chemicals that causes the partial closure of stomatal pores and, thereby, regulates the transpiration process.
1. Healthy leafy twig
2. Phenyl mercuric acetate solution (10 2 M Stock solution)
3. Quick-fix.
4. Conical flasks, beakers etc.
5. Oil, water, graph paper etc.
6. Balance with weight box
1. Prepare 4 sets of transpiration apparatus (conical flask-water-oil-leaf) using fresh petiolate leaves (leaves are cut under water).
2. Treat each set separately by spraying water (as control) or different concentrations of phenyl mercuric acetate solution (10 -3 M, 10 -4 M, 10 -5 M) on both surfaces of leaf.
3. Place the experimental sets under sunshine for 2 hrs.
4. Record the initial and final weights before and after transpiration in each set to determine the amount of water transpired by the leaf of each set.
5. Calculate the rate of transpiration for each set separately.
- Bacteria Lab Report
- Gram Staining
- Reproduction
- Effects on Humans
- Hydroponics Lab Report
- Transpiration Lab Report
- Rat & Pig Dissections
- Spittal Pond Lab Report
- Nonsuch Island
Transpiration Lab Report

IMAGES
COMMENTS
Introduction. In plants, water is transported from the roots to the leaves, following a decreasing water potential gradient. Transpiration, or loss of water from the leaves, helps to create a lower osmotic potential in the leaf. The resulting transpirational pull is responsible for the movement of water from the xylem to the mesophyll cells ...
Introduction. Most of the water a plant absorbs is not used for a plant’s daily functioning. It is instead lost through transpiration, the evaporation of water through the leaf surface and stomata, and through guttation, which is the loss of water from the vascular tissues in the margins of leaves.
ADVERTISEMENTS: The below mentioned article includes a collection of thirteen experiments on transpiration. 1. Experiment to demonstrate the transpiration phenomenon with the bell jar method: ADVERTISEMENTS: Requirements: Bell jar, well-watered potted plant, rubber sheet, glass plate, Vaseline. Method: 1. Take a well-watered, healthy potted plant and cover the pot with the help of rubber sheet
Jun 22, 2023 · Transpiration Experiments: In the article, we will learn about transpiration in plants’ experiments through jar bells and polythene bags, etc. Plants absorb the water through roots, and the xylem transports the water to the stem, leaves, and other parts of the plant. The leaves utilise only about 2 % of the absorbed water in photosynthesis.
Lab 11 Rates of Transpiration Abstract This experiment was conducted to measure the rates of transpiration between 3 variables (Control, Wind, Light, Heat). Each plant was to be monitored and massed over 48 hours. After 48 hours the mass lost was divided by the surface area and this was measured through a T‐Test to see if there were differences.
Feb 13, 2024 · 1. Experiment Introduction & Background Research: Introduction: Plant transpiration is a crucial physiological process where water is absorbed by plant roots, transported through the plant, and eventually released into the atmosphere through stomata in the leaves. This experiment aims to investigate the factors influencing plant transpiration.
III. Plant Transpiration - Experiment: 1. Introduction: 1.) pp. C77-C79: Read the descriptions for transpiration through different tissues and transpiration rate 2.) p. C79: Answer questions 1, 2, 6, and 7 3.) pp. 114-115, Demonstration: View the demo preps of a celery stem ("stalk") and a carnation stem that have been sitting in colored water.
hundred gallons of water through transpiration on a hot, dry day. The rate of transpiration also depends on the type of plant. Succulent plants have much slower transpiration rates because of a thick, waxy coating on the leaves. That thick, waxy coating is why succulent plants are well adapted to live in arid regions like deserts with minimal ...
ADVERTISEMENTS: List of top nine experiments on transpiration in plants:- 1. Measurement of Leaf Area 2. Demonstration of Transpirational Water Loss by Potometers 3. Determination of the Rate of Transpiration by Simple Method (Conical Flask Method) 4. Determination of the Rates of Stomatal and Cuticular Transpiration and a few others. Experiment # 1. Measurement of […]
Transpiration Lab Report Introduction & Hypothesis Transpiration is the loss of water by evaporation in terrestrial plants , especially through the stomata (accompanied by a corresponding water uptake from the roots ); a process in which the water vapor escapes through the plant via its stomata into its external environment .