

Study Largely Confirms Known, Rare COVID-19 Vaccine Side Effects
By Kate Yandell
Posted on February 27, 2024
SciCheck Digest
An international study of around 99 million people confirmed known serious side effects of COVID-19 vaccination. It also identified a possible relationship between the first dose of the Moderna vaccine and a small risk of a neurological condition. Social media posts about the study left out information on the vaccines’ benefits and the rarity of the side effects.
More than half a billion doses of COVID-19 vaccines have now been administered in the U.S. and only a few, very rare, safety concerns have emerged. The vast majority of people experience only minor, temporary side effects such as pain at the injection site, fatigue, headache, or muscle pain — or no side effects at all. As the Centers for Disease Control and Prevention has said , these vaccines “have undergone and will continue to undergo the most intensive safety monitoring in U.S. history.”
A small number of severe allergic reactions known as anaphylaxis, which are expected with any vaccine, have occurred with the authorized and approved COVID-19 vaccines. Fortunately, these reactions are rare, typically occur within minutes of inoculation and can be treated. Approximately 5 per million people vaccinated have experienced anaphylaxis after a COVID-19 vaccine, according to the CDC.
To make sure serious allergic reactions can be identified and treated, all people receiving a vaccine should be observed for 15 minutes after getting a shot, and anyone who has experienced anaphylaxis or had any kind of immediate allergic reaction to any vaccine or injection in the past should be monitored for a half hour. People who have had a serious allergic reaction to a previous dose or one of the vaccine ingredients should not be immunized. Also, those who shouldn’t receive one type of COVID-19 vaccine should be monitored for 30 minutes after receiving a different type of vaccine.
There is evidence that the Pfizer/BioNTech and Moderna mRNA vaccines may rarely cause inflammation of the heart muscle (myocarditis) or of the surrounding lining (pericarditis), particularly in male adolescents and young adults .
Based on data collected through August 2021, the reporting rates of either condition in the U.S. are highest in males 16 to 17 years old after the second dose (105.9 cases per million doses of the Pfizer/BioNTech vaccine), followed by 12- to 15-year-old males (70.7 cases per million). The rate for 18- to 24-year-old males was 52.4 cases and 56.3 cases per million doses of Pfizer/BioNTech and Moderna vaccines, respectively.
Health officials have emphasized that vaccine-related myocarditis and pericarditis cases are rare and the benefits of vaccination still outweigh the risks. Early evidence suggests these myocarditis cases are less severe than typical ones. The CDC has also noted that most patients who were treated “responded well to medicine and rest and felt better quickly.”
The Johnson & Johnson vaccine has been linked to an increased risk of rare blood clots combined with low levels of blood platelets, especially in women ages 30 to 49 . Early symptoms of the condition, which is known as thrombosis with thrombocytopenia syndrome, or TTS, can appear as late as three weeks after vaccination and include severe or persistent headaches or blurred vision, leg swelling, and easy bruising or tiny blood spots under the skin outside of the injection site.
According to the CDC, TTS has occurred in around 4 people per million doses administered. As of early April , the syndrome has been confirmed in 60 cases, including nine deaths, after more than 18.6 million doses of the J&J vaccine. Although TTS remains rare, because of the availability of mRNA vaccines, which are not associated with this serious side effect, the FDA on May 5 limited authorized use of the J&J vaccine to adults who either couldn’t get one of the other authorized or approved COVID-19 vaccines because of medical or access reasons, or only wanted a J&J vaccine for protection against the disease. Several months earlier, on Dec. 16, 2021 , the CDC had recommended the Pfizer/BioNTech and Moderna shots over J&J’s.
The J&J vaccine has also been linked to an increased risk of Guillain-Barré Syndrome, a rare disorder in which the immune system attacks nerve cells. Most people who develop GBS fully recover, although some have permanent nerve damage and the condition can be fatal.
Safety surveillance data suggest that compared with the mRNA vaccines, which have not been linked to GBS, the J&J vaccine is associated with 15.5 additional GBS cases per million doses of vaccine in the three weeks following vaccination. Most reported cases following J&J vaccination have occurred in men 50 years old and older.
Link to this
COVID-19 vaccines — like all vaccines and other medical products — come with side effects, including serious side effects in rare cases. The vaccines were rolled out to protect people from a novel virus that has killed millions of people globally and would likely have killed millions more without the arrival of the vaccines. There is a broad consensus from experts and governmental health agencies that the benefits of COVID-19 vaccination outweigh the risks.
Researchers have scrutinized the COVID-19 vaccines’ safety and continue to do so. A study published Feb. 12 in the journal Vaccine reported on an international group of more than 99 million people who received COVID-19 vaccines, primarily finding links to known rare side effects. The study largely focused on the Pfizer/BioNTech and Moderna vaccines, which have been widely given in the U.S., as well as the AstraZeneca vaccine, which was never authorized in the U.S.

“What we take away, is that the Covid-19 vaccination campaigns have been very effective in preventing severe disease,” study co-author Anders Hviid , head of the department of epidemiology research at the Statens Serum Institut in Denmark, told us in an email. “The few serious side effects that we have observed in this and other studies have been rare.”
Many popular posts on social media have shared results from the study, some lacking the context that the identified health problems are rare , that most aren’t new and that the vaccines have proven benefits. Various posts made unfounded claims, stating or implying that people should not have received the vaccines , that the risks outweigh the benefits or that the risk of the rare side effects is greater than was reported in the study.
“Hundreds of millions of people were used as lab rats and now the truth that WE ALL ALREADY KNEW can no longer be denied,” said one popular post , referring to the vaccines as “experimental” and “UNTESTED.” The post shared a screenshot of the headline of a New York Post article about the new study, which read, “COVID vaccines linked to slight increases in heart, brain, blood disorders: study.”
“This thing was forced on people who faced almost no risk from Covid,” said another widely read post . “It is completely unacceptable.” The post shared statistics from the paper without making it clear that serious health problems after vaccination were rare and that risk varied by vaccine type and dose.
The Vaccine study confirmed that the Moderna and Pfizer/BioNTech vaccines are linked in rare cases to myocarditis and pericarditis, conditions involving inflammation of the heart muscle and lining. The rate of myocarditis was most elevated after the second dose of the Moderna vaccine. Myocarditis risk — which is greatest in men in their late teens and early twenties — was identified via vaccine safety monitoring and first reported in 2021. Based on the current evidence, the CDC says, the benefit of vaccination outweighs the risk of these conditions, which improve for most people after medical treatment and rest.
The study confirmed neurological and blood clotting conditions associated with the AstraZeneca vaccine. In the U.S., these problems were linked to the Johnson & Johnson vaccine, contributing to this vaccine no longer being recommended or available.
The study also identified a new possible safety signal indicating a potential link between the first dose of the Moderna and AstraZeneca vaccines and rare neurological conditions. This included an association between the first doses of the vaccines and acute disseminated encephalomyelitis, or ADEM, an autoimmune condition that causes inflammation of the brain and spinal cord.
Hviid emphasized that the researchers only saw these neurological events after first doses of the two vaccines. “We did not see these signals following further doses of these two Covid-19 vaccines, nor did we see them after any dose of the Pfizer/BioNTech vaccine which has been more widely used,” he said.
“We are also talking about very rare events,” Hviid continued. “As an example, the association between the first dose of Moderna and acute inflammation of the brain and spine would, if causal, correspond to 1 case per 1.75 million vaccinated. It is only due to the sheer scale of our study, that we have been able to identify this minute potential risk.”
Study Bolsters the Evidence Serious COVID-19 Vaccine Side Effects Are Rare
The Vaccine study drew on national or regional health records from eight countries with institutions participating in the Global Vaccine Data Network , an international group that studies vaccine safety. The researchers analyzed health outcomes after around 184 million doses of the Pfizer/BioNTech vaccine, 36 million doses of the Moderna vaccine and 23 million doses of the AstraZeneca vaccine.
The researchers focused on 13 health problems that either had a known association with vaccination or for which there was some rationale to investigate whether there was an association. To determine whether the health problems were associated with vaccination, they compared the expected rates of the health problems — or the number of health events that should occur based on background rates in the regions studied — with the number of events they observed in the 42 days after vaccination.
“This study confirms the primary already detected and validated side effects established by previous literature,” Jeffrey S. Morris , director of the division of biostatistics at the University of Pennsylvania’s Perelman School of Medicine, told us via email, referring to the rare heart conditions associated with the Moderna and Pfizer/BioNTech vaccines, as well as the rare conditions associated with the AstraZeneca and Johnson & Johnson vaccines.
Morris said that findings on ADEM — the rare autoimmune neurological condition linked to first doses of the Moderna and AstraZeneca vaccines — “might be a new safety signal.”
ADEM involves inflammation to the brain and spinal cord, arising most often in children following an infectious illness. It has a sudden onset and typically eventually improves, with a full recovery in many, although not all, cases.
After the first dose of the Moderna vaccine, researchers observed seven ADEM cases, when they expected two. As we’ve said, Hviid calculated the rate of this side effect — if ultimately shown to be related to vaccination — to be 1 in 1.75 million following the first dose of the Moderna vaccine.
The data show “this was indeed an EXTREMELY rare adverse event,” Morris said, referring to ADEM. “It is understandable at this incidence rate why it may not have been detected before now, and why a study with 99 million participants like this is important to find even the most rare serious adverse events that are potential minority harm risks of these vaccines.”
The authors of the study wrote that more research is needed into ADEM following COVID-19 vaccination, saying that “the number of cases of this rare event were small and the confidence interval wide, so results should be interpreted with caution and confirmed in future studies.” The authors also wrote that neurological events have been found to occur at a much higher rate after COVID-19 than after COVID-19 vaccination.
The study means that “early warning systems are solid,” said Marc Veldhoen , an immunologist at the Instituto de Medicina Molecular João Lobo Antunes in Portugal, in a post on X, formerly known as Twitter. “To avoid any adverse reaction is not possible, but, identifying those at higher risk may be possible.”
Identifying those at greater risk of side effects can help guide decisions on which vaccines to recommend and what problems doctors should watch for in their patients.
Editor’s note: SciCheck’s articles providing accurate health information and correcting health misinformation are made possible by a grant from the Robert Wood Johnson Foundation. The foundation has no control over FactCheck.org’s editorial decisions, and the views expressed in our articles do not necessarily reflect the views of the foundation.
“ How do we know vaccines are safe? ” FactCheck.org. Updated 8 Jul 2021.
“ Selected Adverse Events Reported after COVID-19 Vaccination .” CDC website. Updated 12 Sep 2023.
Yandell, Kate. “ Tucker Carlson Video Spreads Falsehoods on COVID-19 Vaccines, WHO Accord .” FactCheck.org. 13 Jan 2024.
“ Safety of COVID-19 Vaccines .” CDC website. 3 Nov 2023.
“ How safe are the COVID-19 vaccines? ” FactCheck.org. Updated 17 May 2022.
Faksova, K. et al. “ COVID-19 Vaccines and Adverse Events of Special Interest: A Multinational Global Vaccine Data Network (GVDN) Cohort Study of 99 Million Vaccinated Individuals .” Vaccine. 12 Feb 2024.
COVID Data Tracker. “ COVID-19 Vaccinations in the United States .” CDC website. Updated 11 May 2023.
Liu, Angus. “ AstraZeneca withdraws US COVID vaccine application, shifts focus to antibody treatments .” Fierce Pharma. 10 Nov 2022.
Hviid, Anders. Email with FactCheck.org. 22 Feb 2024.
TheBlaze. “ Blood clots, neurological disorders, and swollen hearts: Multinational study on COVID vaccines paints a damning picture .” Facebook. 20 Feb 2024.
Dr. Anthony G. Jay (@anthonygjay). “ I post a lot of vids but rarely PLUG them WATCH my YouTube vid on this – it’s 6 minutes – before it gets taken down 🤐 .” Instagram. 20 Feb 2024.
bikinibottom_fish 🐟 (@bikinibottom_fish). “ Global Study Links COVID-19 Vaccines to Heart and Brain Issues! ” Instagram. 20 Feb 2024.
PatrioticBabe 🇺🇸 (@babedoesthenews). “ ❗️ .” Instagram. 20 Feb 2024.
RASPY RAWLS (@raspy_rawls2). “ … We told yall not to take that shyt but hey wat dew we know 🤷🏾♂️ … .” Instagram. 20 Feb 2024.
Jaimee Michell (@thegaywhostrayed). “ I want to know if you think Trump holds any blame, and if not, why not? COMMENT your thoughts BELOW! ” Instagram. 20 Feb 2024.
Liberty Counsel (@libertycounsel). “ … “Based on ‘conservative assumptions,’ the estimated harms of the COVID-19 mRNA vaccines ‘greatly outweigh the rewards,’ the article stated, noting that ‘for every life saved, there were nearly 14 times more deaths caused by the modified mRNA injections.’” … ” Instagram. 20 Feb 2024.
Shemeka Michelle (@ theshemekamichelle ). “ Remember when they called them “rare” breakthrough cases? Yeah, me too. #slight .” Instagram. 20 Feb 2024.
Mal’aki (@awake.the.mind). “ ‘Slight’ will turn to ‘significant’ soon enough. We tried to warn you all but we’re just crazy conspiracy theorists .” Instagram. 20 Feb 2024.
Steinbuch, Yaron. “ COVID vaccines linked to slight increases in heart, brain, blood disorders: study .” New York Post. 20 Feb 2024.
Vogel, Gretchen and Couzin-Frankel, Jennifer. “ Israel reports link between rare cases of heart inflammation and COVID-19 vaccination in young men .” Science. 1 Jun 2021.
Robertson, Lori and Kiely, Eugene. “ Q&A on the Rare Clotting Events That Caused the J&J Pause .” FactCheck.org. Updated 6 May 2022.
Kahn, Ilana. “ Acute Transverse Myelitis and Acute Disseminated Encephalomyelitis .” Pediatrics in Review. 1 Jul 2020.
Morgan, Hannah J. et al. “ Acute Disseminated Encephalomyelitis and Transverse Myelitis Following COVID-19 Vaccination – A Self-Controlled Case Series Analysis .” Vaccine. 12 Feb 2024.
“ Global COVID Vaccine Safety (GCoVS) .” Global Vaccine Data Network website. Accessed 23 Feb 2024.
Morris, Jeffrey S. Email with FactCheck.org. 22 Feb 2024.
Frontera, Jennifer A. et al. “ Neurological Events Reported after COVID-19 Vaccines: An Analysis of VAERS .” Annals of Neurology. 2 Mar 2022.
Marc Veldhoen (@Marc_Veld). “ COVID-19 vaccines and adverse events of special interest: A multinational Global Vaccine Data Network (GVDN) cohort study of 99 million vaccinated individuals Anything in those anti-vax stories about large scale damage and deaths due to vaccines? No. … ” X. 19 Feb 2024.
- Free In-Person Regional Events Focused on Oncology and Population Health

- Conferences
Pfizer COVID-19 Vaccine Effective in Children Aged 5 to 17 Years
Key takeaways.
- The BNT162b2 XBB vaccine shows 68% effectiveness in children aged 5-11 and 63% in those aged 12-17 against severe COVID-19 outcomes.
- The study involved 15,233 children, with a diverse demographic, and used a test-negative case-control analysis to assess vaccine efficacy.
- Increased vaccine uptake in children could prevent thousands of hospitalizations and emergency visits, alleviating healthcare resource strain.
- Limitations include potential confounding factors, misclassification of prior infections, and limited generalizability of the study results.
New research showed that the BNT162b2 XBB vaccine was capable of reducing hospitalizations and emergency department and urgent care visits due to COVID-19 in children aged 5 to 17 years.
A new research letter published in JAMA Network Open 1 highlighted the results of new research which found that the BNT162b2 XBB vaccine, produced by Pfizer Pharmaceuticals, was able to effectively protect children aged 5 to 17 years from severe COVID-19 , reducing both associated hospitalizations and emergency department (ED) or urgent care visits.
Vaccines classified as XBB messenger RNA vaccines for COVID-19 have been found to be effective for preventing mild to severe outcomes in adults in the past, but the effectiveness of the vaccine in children, specifically the updated vaccines, has been less researched. Severe outcomes of COVID-19 could lead to hospitalization of a patient, which put a strain on health care resources during the height of the pandemic. 2 Therefore, preventing mild and severe symptoms can help to prioritize those in greater need of immediate care. Testing the efficacy of the BNT162b2 XBB vaccine in children is a method of reducing hospital resource spending as well as helping to avoid long-term consequences of COVID-19 in this demographic.
The BNT162b2 XBB COVID-19 vaccine was found effective in children aged 5 to 17 years | Image credit: Leigh Prather - stock.adobe.com
This study was a test-negative case-control analysis. The analysis was evaluating the effectiveness of the vaccine against hospital admission and ED or urgent care visits related to acute respiratory infection (ARI) between October 10, 2023, and April 30, 2024. Children aged 5 to 11 years receiving the 10-μg vaccine and children aged 12 to 17 years receiving the 30-μg vaccine were included in the study from the Kaiser Permanente Southern California database. All of the patients included had a polymerase chain reaction or antigen test for detecting COVID-19 during a hospital admission or ED or urgent care visit.
There were 15,233 children included in the study, of which 9834 were children aged 5 to 11 years. Most of the participants were Hispanic (57.5%), followed by White (17.6%), Asian or Pacific Islander (9.5%), and Black (8.9%). A total of 48.2% of the participants were female.
There were 1125 children, 339 aged 5 to 11 years and 264 aged 12 to 17 years, who received the vaccine by April 2024, with a median (range) time since vaccination of 75 (15-199) days and 64.5 (16-197) days respectively. The estimated vaccine effectiveness was 68% (95% CI, 11%-88%) in children aged 5 to 11 years and 63% (95% CI, 20%-83%) in children aged 12 to 17 years. The efficacy was measured against hospital admissions or ED or urgent care visits related to COVID-19. The overall effectiveness for all children was approximately 65% (95% CI, 36%-81%), with no hospitalizations due to COVID-19 reported in any child who received the vaccine.
“Assuming 65% vaccine effectiveness, vaccinating the roughly 54.3 million 5-to-17-year olds in the US could have averted approximately 3700 hospitalizations and…roughly 111,000 ED or urgent care visits during the 2023-2024 respiratory virus season,” the authors wrote.
There were some limitations to this research, including the potentially unmeasured confounding factors, potential misclassification of prior infection or whether encounters with ARI were truly related to COVID-19, and a lack of generalizability. The results of this study suggest that improving COVID-19 vaccine uptake in the pediatric population should be top priority to avoid severe symptoms of COVID-19 in this population.
- Tartof SY, Frankland TB, Puzniak L, et al. BNT162b2 XBB vaccine for COVID-19 among children 5-17 years of age. JAMA Netw Open . 2024;7(12):e2449944. doi:10.1001/jamanetworkopen.2024.49944
- French G, Hulse M, Nguyen D, et al. Impact of hospital strain on excess deaths during the COVID-19 pandemic—United States, July 2020-July 2021. Am J Transplant . 2022;22(2):654-657. doi:10.1111/ajt.16645

5 Things to Know About COVID-19 Before the Holiday Season

Addressing the Demand for Mental Health Services During the COVID-19 Pandemic
COVID-19 Infection Increases Risk of Severe Outcomes in Patients With Certain Cardiomyopathies

Promoting Equitable Access to Telemedicine During the COVID-19 Crisis
Key COVID-19 Recommendations for 2024-2025 Respiratory Virus Season

5 Things You Should Know About Long COVID

2 Commerce Drive Suite 100 Cranbury, NJ 08512
© 2024 MJH Life Sciences ® and AJMC® . All rights reserved.

- - Google Chrome
Intended for healthcare professionals
- Access provided by Google Indexer
- My email alerts
- BMA member login
- Username * Password * Forgot your log in details? Need to activate BMA Member Log In Log in via OpenAthens Log in via your institution

Search form
- Advanced search
- Search responses
- Search blogs
- News & Views
- Covid-19: Pfizer...

Covid-19: Pfizer vaccine efficacy was 52% after first dose and 95% after second dose, paper shows
Read our latest coverage of the coronavirus outbreak.
- Related content
- Peer review
- Elisabeth Mahase
The Pfizer and BioNTech covid-19 vaccine may provide some early protection, starting 12 days after the first dose, the peer reviewed results of a phase III trial have found.
The study, published in the New England Journal of Medicine , 1 found that vaccine efficacy between the first and second doses was 52% (95% credible interval 29.5% to 68.4%), with 39 cases of covid-19 in the vaccine group and 82 cases in the placebo group.
Seven or more days after the second dose, vaccine efficacy then rose to 95% (90.3% to 97.6%), with eight covid-19 cases reported in the vaccine group and 162 cases in the placebo group.
The vaccine has so far been approved in Canada and in the UK, where it is already being rolled out to people over 80 and healthcare workers. In the US the Food and Drug Administration’s independent panel has voted in favour of emergency use authorisation for the vaccine, and the agency is expected to approve it within days. 2
Participants
From July to November 2020, 43 448 adults were randomly assigned at 152 sites worldwide (including in Argentina, Brazil, Germany, South Africa, Turkey, and the US) as part of the phase II/III trial of the BNT162b2 vaccine. A total of 21 720 people received two doses 21 days apart, and 21 728 received a placebo.
The paper reported that, seven days after the second dose, vaccine efficacy ranged from 89% to 100% across subgroups defined by age, sex, race, ethnicity, baseline body mass index, and the presence of coexisting conditions.
The study found 10 severe covid-19 cases after the first dose, nine of which were in the placebo group. After the second dose it showed one case in the vaccine group and four in the placebo group. 3
As of 9 October, 37 706 participants had a median of at least two months’ safety data available after a second dose. Among these participants 49% were female, 83% were white, 9% were black or African-American, 28% were Hispanic or Latinx, 35% had a body mass index of at least 30, and 21% had at least one pre-existing condition. The median age was 52, and 42% of participants were aged over 55.
Adverse events
In terms of safety, more people in the covid-19 vaccine group reported any adverse event (27%, compared with 12% taking a placebo) or a related adverse event (21% v 5%). The researchers said that this was mainly due to transient reactogenicity events, such as injection site pain.
Few participants in either group had severe or serious adverse events. Among the BNT162b2 recipients four related serious adverse events were reported and two recipients died (one from arteriosclerosis and one from cardiac arrest), as did four placebo recipients (two from unknown causes, one from haemorrhagic stroke, and one from myocardial infarction). However, none of the deaths was considered by the investigators to be related to the vaccine or placebo, and no covid-19 associated deaths were observed.
The researchers wrote, “The safety profile of BNT162b2 was characterised by short term, mild-to-moderate pain at the injection site, fatigue, and headache. The incidence of serious adverse events was low and was similar in the vaccine and placebo groups.”
This article is made freely available for use in accordance with BMJ's website terms and conditions for the duration of the covid-19 pandemic or until otherwise determined by BMJ. You may use, download and print the article for any lawful, non-commercial purpose (including text and data mining) provided that all copyright notices and trade marks are retained.
- ↵ Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med 2020. doi: 10.1056/NEJMoa2034577 . https://www.nejm.org/doi/full/10.1056/NEJMoa2034577?query=RP .
- ↵ Polack FP, Thomas SJ, Kitchin N, et al. Supplementary appendix to “Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine.” N Engl J Med 2020. https://www.nejm.org/doi/suppl/10.1056/NEJMoa2034577/suppl_file/nejmoa2034577_appendix.pdf .
- Editors' picks
- Culture & Trends
- Share & Save —
- Decision 2024
- Investigations
- Tech & Media
- Video Features
- NBC Asian America
- Los Angeles
- Dallas-Fort Worth
- Philadelphia
- Washington, D.C.
- South Florida
- Connecticut
- Nightly News
- Meet the Press
- NBC News Now
- Nightly Films
- Special Features
- Newsletters
More From NBC
- NBCU Academy
- NEXT STEPS FOR VETS
- NBC News Site Map
Follow NBC News
news Alerts
There are no new alerts at this time
- Latest Stories
Two years after Covid vaccines rolled out, researchers are calling for newer, better options
Two years after the first Covid shots went into arms, a growing chorus of researchers is calling for a new generation of vaccines that provide broader and more long-term protection against the disease.
The U.S. is currently recording around 430 Covid deaths per day, on average, according to NBC News’ tally . That includes many people who received at least two Covid shots: Six in 10 adults who died of Covid in August were vaccinated or boosted, according to a report by KFF , a nonprofit health think tank. And for the most part, vaccinated people don’t avoid infections or reinfections anymore.
“Coming up with a vaccine that’s going to last longer and cover a wider range of the Covid family of viruses is a life and death problem,” said Dr. Tom Frieden, who directed the Centers for Disease Control and Prevention until 2017 and is now president of the public health organization Resolve to Save Lives.
Many people thought the solution to that problem had arrived two years ago, on December 14, 2020, when Sandra Lindsay became the first person in the U.S. to get a Covid vaccine outside of a clinical trial.
“My whole life just changed tremendously in that one moment in time,” said Lindsay, who is now the vice president of public health advocacy at Northwell Health.
“What was going through my mind is, ‘I cannot wait for this needle to pierce my arm,’” she said.
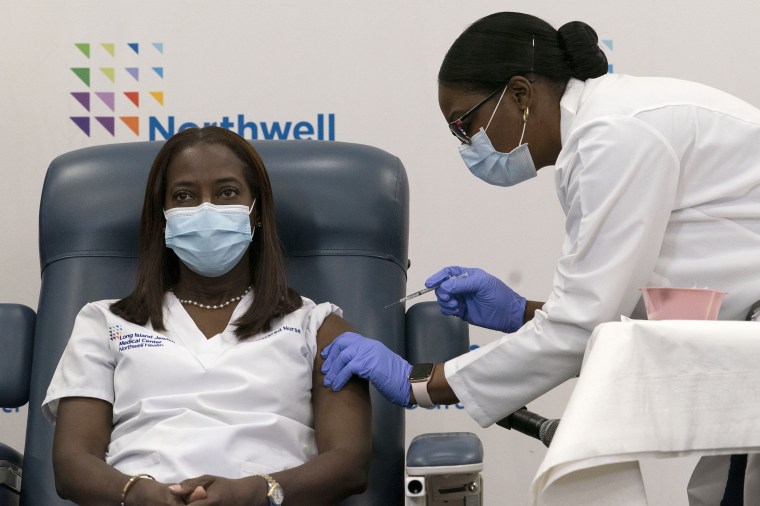
Millions of people shared her impatience, for good reason: Adults who are up to date on their shots are 15 times less likely to die from Covid than those who are unvaccinated. Covid vaccines prevented more than 3.2 million deaths and 18.5 million hospitalizations in the U.S. from December 2020 through November 2022, according to an analysis published Tuesday by the Commonwealth Fund and Yale School of Public Health.
But at first, the shots were perceived to be even more powerful than that — a shield against mild symptoms and a ticket back to pre-pandemic life. The reality proved more complicated and, in certain ways, disappointing.
Many experts maintain that we can — and must — do better.
In particular, researchers think sprays or drops given through the nose or mouth could do a better job of stopping transmission. They also hope that vaccines that target multiple parts of the virus or several variants at once could reduce the need for continuous boosters.
“It’s sometimes easy to forget what a tremendous achievement it was to get a brand new vaccine against a brand new class of viruses. … It was pragmatic, and it was tremendously successful. But it’s certainly not the panacea,” said Matthew Miller, scientific director of the Michael G. DeGroote Institute for Infectious Disease Research at McMaster University. “We can certainly improve on what we know now.”
The promises and shortcomings of mRNA
Vaccine researchers generally agree that mRNA technology was suited to the needs of this pandemic, since it allowed scientists to develop a vaccine quickly at a time when each day meant more lives lost. Scientists then updated the shots relatively easily to target new variants.
“If it wasn’t mRNA, it wouldn’t have gotten done so fast,” said Dr. Barney Graham, former deputy director of the National Institutes of Health’s Vaccine Research Center, now a senior adviser for global health equity at Morehouse School of Medicine.
To date, he added, the Covid vaccine is “one of our simplest, safest vaccines that we’ve ever made.”
When Lindsay got her vaccine in 2020, she was dealing with severely ill Covid patients every day as director of nursing critical care at Long Island Jewish Medical Center.
“It felt like you were just walking into a burning building, but it’s your job,” she said. “It’s what I love doing: taking care of people. So I was going in there no matter what, and just praying every day that I don’t fall ill.”

Despite the odds, Lindsay still hasn’t gotten Covid, as far as she knows. But the majority of Americans have, according to CDC estimates — a situation most of the public did not anticipate when clinical trial results showed 95% efficacy against symptomatic disease.
“It may be that the vaccines were their own worst enemies in some ways, because they were so good initially that people had an expectation that went beyond reason,” Graham said.
Experts agree now, though, that protection from Covid shots fades too fast. Plus, a lack of access to vaccines in many countries allowed the virus to spread rampantly and mutate over time, which has undermined vaccines and treatments.
“If we had immunized the whole world in six months, we may not be having all the problems with the variants because we would have constrained [the] virus’ spread earlier,” Graham said.
The future of Covid vaccines: No needles
When Lindsay volunteered to get her vaccine on day one, she wasn’t aware that she was the country’s very first recipient — despite the cameras.
Now, she said, she still gets recognized.
“I was in TJ Maxx the other day and this man who I didn’t expect was basically bowing down at my feet, [saying] that through my one action, I saved his life, his family’s lives, and so many more,” Lindsay said. “Those are the stories that just solidify for me that what I did on that day made a big difference.”
But others see Lindsay as the face of a promise that fell short.
“You get this on social media, when you post anything: ‘Well, how do you feel now that this thing was all a lie? People are not supposed to get Covid and you got the shots and it’s a big letdown,’” she said.

Researchers still hope that in the future, nasal spray vaccines could inspire more confidence by offering more protection against illness, reducing side effects and removing needles from the equation.
Because Covid seems to infect most people through the nasal passages first, administering a vaccine in the nose could vanquish the virus before it has a chance to spread, the thinking goes.
“It’s sort of akin to having guards placed outside the door in the mucus layer, versus waiting for the invaders to come in,” said Dr. Akiko Iwasaki, an immunobiology professor at Yale University who is developing an intranasal Covid vaccine.
Globally, 117 intranasal Covid vaccines are in development or have been rolled out, according to an analysis provided to NBC News by Airfinity , a health analytics company. Five have been approved in at least one country — two in China and one each in India, Iran and Russia — and 20 more have entered clinical trials. The majority rely on traditional vaccine platforms, not mRNA.
“There’s probably a number of advantages to the intranasal route that will be realized once that route is fully exploited. People can administer it themselves. You can use it in a developing world setting. You can use lower doses,” said Dr. David Curiel, a professor of radiation oncology at Washington University School of Medicine in St. Louis. “There may even be a safety gain, and you get sterilizing immunity and possibly block transmission.”
Curiel developed the technology for the nasal vaccine approved in India. But the vaccine hasn’t entered trials in the U.S., and trial results from India haven’t been published in a peer-reviewed journal.
Other researchers are betting on inhaled vaccines, which come in the form of aerosolized mists administered through a nebulizer into the lungs, where the virus tends to wreak the most havoc.
In September, China approved an inhaled version of a previously authorized Covid shot, called Convidecia. A small trial showed that the inhaled booster dose produced a stronger antibody response than a booster of the intramuscular shot.
Miller and his McMaster colleagues are testing two inhaled vaccines in phase 1 human trials. The more effective candidate will likely advance to phase 2, he said.
Those vaccines might offer an additional advantage, according to Miller: They target three parts of the coronavirus, whereas the current shots target just the spike protein, which mutates faster than the virus’ other components.
Still, some researchers worry that protection from nasal or inhaled vaccines could also wane quickly.
“If we can give a vaccine at the site where infection typically occurs, we would always love to do it that way. The challenge, of course, is that sometimes it doesn’t generate the same type of bloodstream immunity that we really want,” said Dr. Buddy Creech, director of the Vanderbilt Vaccine Research Program.
Creech said future versions of mRNA shots could potentially be tweaked to target three or more coronavirus strains. (The new bivalent boosters target two.)
“It will not be surprising if at some point we need something like a trivalent vaccine or some other permutation of what we have now,” he said. “It could very well mirror what we do with influenza.”

Then there’s the idea of targeting several different coronaviruses at once. The National Institute of Allergy and Infectious Diseases has allocated more than $62 million for research and development of pan-coronavirus vaccines.
In July, researchers at the California Institute of Technology showed that their candidate protected mice and monkeys from the viruses that cause both Covid and SARS. In October, researchers at Duke University School of Medicine similarly showed that their pan-coronavirus vaccine protected animals from multiple SARS-related viruses.
But unlike in 2020, the federal government’s motivation to fund Covid-related innovations is drying up. Whatever Covid vaccine comes next is likely three to five years off, Miller estimated — or perhaps longer, according to other experts.
“The mRNA technology is remarkably successful — these vaccines work better than we had the right to expect,” Frieden said. “But the virus is adapting. And as the virus adapts, we need to adapt.”
Aria Bendix is the breaking health reporter for NBC News Digital.

IMAGES
COMMENTS
The Vaccine study confirmed that the Moderna and Pfizer/BioNTech vaccines are linked in rare cases to myocarditis and pericarditis, conditions involving inflammation of the heart muscle and lining ...
Here, we report safety and efficacy findings from the phase 2/3 part of a global phase 1/2/3 trial evaluating the safety, immunogenicity, and efficacy of 30 μg of BNT162b2 in preventing Covid-19 ...
A new research letter published in JAMA Network Open 1 highlighted the results of new research which found that the BNT162b2 XBB vaccine, produced by Pfizer Pharmaceuticals, was able to ...
Pfizer has a rich history in vaccine research and development, playing a pivotal role in eliminating infectious diseases globally. Here's the latest news. ... nonprofits and governments to address the primary scientific barriers to developing new vaccines and immunotherapies. The project has been endorsed by 35 of the world's leading vaccine ...
Data from 43,448 participants, half of whom received BNT162b2 and half of whom received placebo, showed that the vaccine candidate was well tolerated and demonstrated 95% efficacy in preventing COVID-19 in those without prior infection 7 days or more after the second dose Vaccine efficacy observed in the overall study population was also generally consistent across subgroups defined by age ...
Our analyses indicate that vaccine effectiveness generally decreases over time against SARS-CoV-2 infections, hospitalisations, and mortality. The baseline vaccine effectiveness levels for the omicron variant were notably lower than for other variants. Therefore, other preventive measures (eg, face-mask wearing and physical distancing) might be necessary to manage the pandemic in the long term.
Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of the Pfizer-BioNTech and Oxford-AstraZeneca vaccines on covid-19 related symptoms, hospital admissions, and mortality in older adults in ...
Primary efficacy analysis demonstrates BNT162b2 to be 95% effective against COVID-19 beginning 28 days after the first dose; 170 confirmed cases of COVID-19 were evaluated, with 162 observed in the placebo group versus 8 in the vaccine group Efficacy was consistent across age, gender, race and ethnicity demographics; observed efficacy in adults over 65 years of age was over 94% Safety data ...
The Pfizer and BioNTech covid-19 vaccine may provide some early protection, starting 12 days after the first dose, the peer reviewed results of a phase III trial have found. The study, published in the New England Journal of Medicine ,1 found that vaccine efficacy between the first and second doses was 52% (95% credible interval 29.5% to 68.4%), with 39 cases of covid-19 in the vaccine group ...
Sandra Lindsay is inoculated with the Pfizer-BioNTech Covid-19 vaccine by Dr. Michelle Chester at Long Island Jewish Medical Center in Queens, N.Y., on Wednesday. Mark Lennihan / AP Pool file