
FREE K-12 standards-aligned STEM
curriculum for educators everywhere!
Find more at TeachEngineering.org .
- TeachEngineering
- Pop Rockets

Hands-on Activity Pop Rockets
Grade Level: 3 (3-5)
Time Required: 45 minutes
Expendable Cost/Group: US $0.50
Group Size: 3
Activity Dependency: None
Subject Areas: Earth and Space, Physical Science, Science and Technology
NGSS Performance Expectations:

Curriculum in this Unit Units serve as guides to a particular content or subject area. Nested under units are lessons (in purple) and hands-on activities (in blue). Note that not all lessons and activities will exist under a unit, and instead may exist as "standalone" curriculum.
- I'm Not in Range: Acting Out Cellular Phone Service
- Newton Rocket Car
- Strawkets and Thrust
- Strawkets and Weight
- Strawkets and Control
- Fuel Mystery Dis-Solved!
- Aqua-Thrusters!
- Constraints: Pop Rockets on a Shoestring Budget
- Rocket Power
- Our Sun and Heat Transfer Basics: Heat It Up!
- Cooking with the Sun: Comparing Yummy Solar Cooker Designs
- Spacecraft Design: Beat the Heat!
- Designing Ways to Get and Clean Water
- The Great Gravity Escape
- Lunar Lollipops: Reproducing the Moon Phases
- Edible Rovers
- Are We Alone?
- A Roundabout Way to Mars
- Slingshot to the Outer Planets
- Muscles, Muscles Everywhere
- Design Devices to Help Astronauts Eat: Lunch in Outer Space!
- The North (Wall) Star
- Building a Fancy Spectrograph
TE Newsletter
Engineering connection, learning objectives, materials list, worksheets and attachments, more curriculum like this, introduction/motivation, troubleshooting tips, activity extensions, activity scaling, user comments & tips.

Engineers design scale models of projects to learn and experiment with how they perform. When designing rockets, engineers develop small prototypes to test fuel properties. Does the fuel burn too high? Does the fuel create enough thrust? Rocket and engine prototypes help engineers discover the balance between weight and thrust that is necessary for space flight.
After this activity, students should be able to:
- Explain that energy needed for a rocket launch is related to the size of the rocket.
- Collect and analyze data on model rocket launch height, comparing to size or weight of the rocket.
- Describe what factors an engineer must consider when designing a rocket.
Educational Standards Each TeachEngineering lesson or activity is correlated to one or more K-12 science, technology, engineering or math (STEM) educational standards. All 100,000+ K-12 STEM standards covered in TeachEngineering are collected, maintained and packaged by the Achievement Standards Network (ASN) , a project of D2L (www.achievementstandards.org). In the ASN, standards are hierarchically structured: first by source; e.g. , by state; within source by type; e.g. , science or mathematics; within type by subtype, then by grade, etc .
Ngss: next generation science standards - science, common core state standards - math.
View aligned curriculum
Do you agree with this alignment? Thanks for your feedback!
International Technology and Engineering Educators Association - Technology
State standards, colorado - math, colorado - science.
Each student needs:
- 1 35-mm film canister with an internal snapping lid ( available online ) or 1 mini plastic food container with a snap on lid ( available online )
- one-half of an antacid tablet, such as Alka-Seltzer® brand
- 1 sheet of paper
- cellophane tape
- markers or crayons
- Pop Goes the Rocket Quiz
- Rocket Build Instructions
- Rocket Size/Height Worksheet (or Rocket Weight/Height Worksheet )
For the entire class to share
- access to a sink, to obtain and dispose of tap water
- tape or chalk, to mark off the launch area
- safety glasses/goggles, enough pairs for the largest group and instructor
- paper towels, for clean-up
- (if launching inside) pitcher, from which to launch the rockets
Note: For this activity, a film canister with an internal-sealing lid must be used, not one with a lid that snaps over the outside of the rim. These are usually translucent white plastic canisters, not the black plastic ones. Sometimes these film canisters can be obtained for no cost from camera shops and film processing stores (such as grocery stores, Target, Wal-mart, Costco, etc.) where they recycle the canisters and donate them for educational use. You may have to make several trips to obtain enough canisters. Alternatively, purchase the film canisters ($16 for 30 canisters) at https://www.amazon.com/Microlab-Scientific-FCFR-224-Film-Canisters/dp/B00IMUBZFY . Or, instead of film canisters, use mini plastic food storage containers, as long as they have snap-on lids (not twist on), so they work just like the film canister lid; use containers that are small enough so that they are similar in size/volume as the film canisters (1.25-in x 2-in high; 30-mm diameter x 50-mm high). As another idea, use a cork in a bottle, building the rocket on the cork, although this increases the scale of the experiment a bit. The latter two options require some advance experimentation to verify/test/adjust the amount of fuel/antacid that works safely.
Rockets are incredible machines that are designed by engineers and used to explore space. Have you ever seen a rocket or a photograph of one? How do engineers get these heavy vehicles into space? Something very strong is needed to push the rocket upward into the atmosphere and into space. A rocket needs a lot of energy to move.
Let's think about energy. How do we have energy to move our bodies when we get out of bed in the morning or when we walk to school? We get our energy from food; essentially, food is fuel. Well, rockets use propellant, which is a mixture of fuel and an oxidizer to burn the fuel. Large rockets use a lot of propellant in order to create enough energy to reach space. What if we made a model rocket that was very light? Would we need as much energy as a regular rocket? No, probably not.
Antacid tablets have stored chemical energy in them, and this energy can be released when mixed with water. Although it is not much energy, it is enough to launch a small rocket made out of a film canister and paper. And, for our purposes, this particular chemical energy is also a lot safer than burning real fuel.
Engineers build and test rockt models, which they call prototypes , before building the real thing. Doing this helps them in creating the best designs. When designing Tess' rocket, her engineering team must consider many things, such as the weight, cost, thrust and stability of the rocket. We can use small model rockets to test the performance before Tess spends a lot of time building an expensive full-size rocket. By testing small-scale models, engineers make sure rockets will work, without wasting time and money on testing full-size huge rockets. With a scale model, they can test the thrust and stability and make modifications in order to design the best rocket they can. This is what we are going to do today—design model rockets and find out if we can propel them high into the air using simple chemical energy created from Alka-Seltzer® tablets and water.
Before the Activity
- Gather materials and make copies of the Pop Goes the Rocket Quiz , Rocket Build Instructions , and Rocket Size/Height Worksheet (or Rocket Weight/Height Worksheet ; refer to the Activity Scaling section).
- Find an inside or outside wall suitable for students to launch next to, and use tape or chalk to mark off 10 feet at 1-foot intervals.
- Remove antacid tablets from packaging and break them into halves. A half-tablet is sufficient to pop off a film canister lid; too much antacid makes the lid pop off sooner, which is not desirable for this activity.
With the Students
- Divide the class into groups of three or four students each. Give each student a film canister and sheet of paper. Hand out the various attachments, as needed, throughout the activity. (Note: Give out the antacid tablets only as needed so that you know that all tablets are accounted for and used only in the experiment.)
- Direct students to use scissors and tape to follow the instructions to build a rocket. Encourage groups to experiment with different sizes. Tips: Make sure students put the lids of their canisters at the bottom of the rocket (that is, so the canister is inverted.) Also, make sure the canister lid sticks out from the paper a little so that the paper surrounding the rocket does not interfere with the lid snapping on or popping off.
- Have students put their names and any designs on the rocket paper surface.
- One group at a time, have students move to the launch area to prepare to launch. Note: Require students to wear safety glasses/goggles during their launches and put them on BEFORE the launch begins . Have all other students watch from a safe distance away from the launch area, ready to record the results on their worksheets.
- To launch, have a student hold his/her rocket upside down, and carefully fill the canister 1/3 full of water.
NOTE: The next steps must be done quickly:
- One at a time , have a student drop the half-tablet of antacid into his/her film canister.
- Quickly, snap the lid on tightly.
- Very quickly, turn the rocket upright (which means that the film canister lid is down) into the empty pitcher or onto the flat launch site and stand back!
Expect the rocket to pop within 1-5 seconds.
- Ask students to note the maximum height reached by the rockets and have them record this information on their worksheets.
- Give the popped lid back to the student launcher and repeat steps 6-9 for each student in each group.
Pre-Activity Assessment
Concept Inventory: Have students attempt the Pop Goes the Rocket Quiz . Have students answer the first two quiz questions and then it put aside to complete after the activity.
Activity Embedded Assessment
Data Recording: As directed in the Procedure section, have students decide if the rocket is small (S), medium (M) or large (L) before it is launched. Then, after each rocket is launched, have students measure the maximum height reached by the rocket and record it on the appropriate box in their Rocket Size/Height Worksheets . Discuss any patterns in rocket size or weight versus launch height.
Post-Activity Assessment
Pairs Check/Concept Inventory Continued: Have students complete question 3 of the Pop Goes the Rocket Quiz (begun during pre-assessment). After students finish working individually on the quiz, have them compare answers with a peer, giving all students time to finish. Finally, go over the answers as a class.
Survey: Ask the following questions (verbal or written) to survey students about the activity. Have students use what they observed in the activity to help answer the questions.
- What makes one rocket perform better than another? (Answer: Many factors such as weight, drag, thrust [rate of gas build up], canister symmetry, canister seal tightness, and wind can affect rocket performance.)
- What is creating the thrust in our pop-rockets? (Answer: The high pressure built up from the chemical reaction of the antacid tablet and water in the canister forces the cap off and downward as the rocket moves upward—an illustration of Newton's third law of motion.)
- If students previously performed a strawket activity from Lesson 2 of this unit, ask them to compare how these rockets are more "rocket-like" than those launched by a straw? (Answer: Like real rockets, these pop rockets carry their own fuel.)
- How are pop rockets related to real rockets? (Answer: Real rockets behave according to Newton's laws of motion just like pop rockets do. Also, solid propellant rockets have a similar process by releasing energy through a chemical reaction to generate thrust.)
- Use your observations and/or measurements of the motion of the pop rockets to provide evidence that a pattern can be used to predict future motion.
Sales Pitch! Have students pretend to be inventors selling their rockets to a manufacturers or consumers. Have student teams create persuasive posters or flyers, as well as 10-minute sales pitches of their rocket designs for presentation at the next class. Have them include in their advertising a description of where the energy comes from to launch the rocket.
Safety Issues
- Make sure students wear safety glasses/goggles while they are launching their rockets.
- Make sure that students who are not launching stay a safe distance away from the launch area.
- Remind students not to put the antacid tablets in their mouths; if a student eats an entire tablet s/he could become sick.
- Hand out the antacid tablets only at launch time; do not give each group a "supply" in advance.
Common student problems when building rockets:
- Forgetting to tape the film canister to the rocket body.
- Failing to mount the canister with the lid end down.
- Not extending the canister far enough from the paper tube to make snapping on the lid easy.
It may be easier for students to build the rockets and have the teacher launch them since the chemical reaction of the antacid and water sometimes happens too fast for small hands.
Warn students to stand back when the rockets are launching, so that they do not get hit with flying canister parts.
Have students experiment with different amounts of water and tablet sizes. Make sure students wear safety glasses/goggles when launching their rockets.
Have students launch for distance instead of height. Have them measure the launch angle and record data for multiple angles.
For kindergarten and first-grade students, conduct the activity as a demonstration instead of having students individually make their own rockets. Prior to class, make several different-sized rockets with no fins and then have students each color a fin to put on the rockets. Have students count the number of fins on each rocket. Have students count out loud to see how long it takes each rocket to "pop." Instead of using the Pop Goes the Rocket Quiz as an assessment, ask students what geometric shapes they see in the pop rockets. Then, ask for a choral response to these basic questions:
- Why does the rocket come back down when shot up? (Answer: Gravity)
- Where is the energy coming from to power the rocket? (Answer: The antacid table and water reaction.)
For second-grade students, build the rockets as indicated, but help them with the launching procedure, as necessary.
For fourth- and fifth-grade students, also have them measure the mass of their rockets on a scale before launching them. Then, have them calculate the rocket weights using the equation: weight = mass × (acceleration due to gravity). Provide students with the Rocket Weight/Height Worksheet to record their data, instead of the Rocket Size/Height Worksheet.

Students learn how rocket thrust is generated with propellant. The two types of propellants are discussed—liquid and solid—and their relation to their use on rockets is investigated. Students learn why engineers need to know the different properties of propellants.

Your students have been hired to build a pop rocket, but on a tight budget. Engineering design usually has some constraints and you won’t always have access to the materials you think you might need. But through brainstorming and trial and error, a viable rocket launch is definitely possible!

Through the continuing storyline of the Rockets unit, this lesson looks more closely at Spaceman Rohan, Spacewoman Tess, their daughter Maya, and their challenges with getting to space, setting up satellites, and exploring uncharted waters via a canoe. Students are introduced to the ideas of thrust,...

While building and testing model rockets fueled by antacid tablets, students are introduced to the basic physics concepts on how rockets work. Students revise and improve their initial designs.

Fisher, Diane. National Aeronautics and Space Administration. Space Place , "Build a Bubble-Powered Rocket," September 8, 2005. http://spaceplace.nasa.gov/pop-rocket/
The Society for International Space Cooperation. Space Xpress, International Space Station Curriculum and Activities , "Film Canister Rockets." http://www.spacesociety.org/spaceexpress/Curriculum/film_canisters.html
Contributors
Supporting program, acknowledgements.
The contents of this digital library curriculum were developed under grants from the Fund for the Improvement of Postsecondary Education (FIPSE), U.S. Department of Education and National Science Foundation (GK-12 grant no. 0338326). However, these contents do not necessarily represent the policies of the Department of Education or National Science Foundation, and you should not assume endorsement by the federal government.
Last modified: September 6, 2022
Science Fun

Pop Rocket Chemical Reaction Science Experiment
Learn how to make a pop rocket in this quick, super fun, and easy chemical reaction science experiment. This is a new spin on the traditional Alka-Seltzer film canister pop rocket experiment. Since the old film canisters are getting harder to come by, we will use a Mini M&Ms tube to perform this chemical reaction science experiment.
- Empty Mini M&M’s tube. These are readily available at grocery and convenience stores and come in a variety of cool colors.
- Alka-Seltzer tablets
Instructions:
- Find an area outside that is easy to clean up and can get a little messy.
- Look up and make sure there is nothing that can get hit and broken above where you plan to launch your pop rocket.
- Use the scissors to cut the little plastic tab that acts as a hinge for the tube lid.
- Break an Alka-Seltzer tab in half and put it in the tube.
- Fill the tube about 3/4 of the way full with water.
- Quickly snap on the lid and set your pop rocket on the ground upside down (on its lid).
- Step back a few feet.
- Watch and wait for the chemical reaction to cause your pop rocket to launch into the air.
EXPLORE AWESOME SCIENCE EXPERIMENT VIDEOS!

How it Works:
The water causes a chemical reaction to occur when the dry ingredients in the Alka-Selzer tablet, citric acid and sodium bicarbonate, dissolve and create carbon dioxide. The carbon dioxide builds up pressure until it causes the lid to “pop” off and launch the pop rocket into the air.
Make This A Science Project:
Test different amounts of Alka-Seltzer tablet and observe and record any noticeable differences. Try different liquids. Try different containers. Test different amounts of water. Add fins to your tube to see if this cause the pop rocket to travel at a different speed or distance.
EXPLORE TONS OF FUN AND EASY SCIENCE EXPERIMENTS!

SUBSCRIBE AND NEVER MISS A NEW SCIENCE FUN VIDEO!
previous experiment
Next experiment.

Exploding Pop Rockets
This 4th of July twist on those classic Alka-Seltzer pop rockets is a must-try kids’ science experiment your kids will beg to repeat over and over again. The easy kids’ science project is perfect for the 4th of July, New Year’s Eve or just because!
Follow the simple step-by-step below and then grab 30 Science Experiments kids will beg to repeat in our shop!

This kids’ science experiment uses antacids which can contain aspirin so make sure to inform your kids that it is a medicine and should not normally be played with. Also, make sure to clean up any remaining bits of antacid tablets – especially if you have toddlers in your home.
Getting Ready
To prep, I gathered together a few supplies:
- Mini M&M tubes
- Antacid tablets
- Cardboard (cereal boxes work great)
- Glue gun (not pictured)
- Decorations (stickers, washi tape, painters tape)
- Bottle of water
- Small ball of clay (optional)
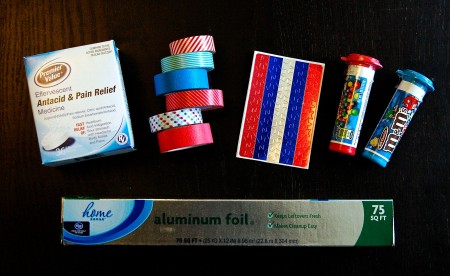
It was time to start this American-themed kids’ science experiment! To build the rockets, I first emptied and peeled the label off the M&M tubes. Next, I cut the tab that holds the cap on the tube so the cap could be completely removed. Then, I used an empty cereal box and my hot glue gun to make mini cones to top off the rockets

I called A over and handed her the stickers and tape so she could decorate her rockets. While she was busy happily peeling stickers, I covered the cones with aluminum foil to finish off the tops and to provide them with a little more protection. When A was done, I hot glued the cones to the bottom (the side opposite the cap) of the M&M tubes and we were ready to make the rockets fly.

We gathered our rockets, the antacid tablets, and a bottle of water and headed outside. This activity can get pretty messy, especially if you are like us and launch your rocket dozens of times. I placed the small ball of clay in the lid to hold the antacid tablet in place so that A could close the lid without starting the fizz until the rocket was flipped right side up.

Next, I poured about a teaspoon of water into the rocket and handed the rocket and lid to a nervous A. She placed the lid snugly on the rocket, flipped the rocket onto the wooden board I had placed on the grass and ran for cover.

The first reactions happened quickly giving A only a few seconds to run away before it popped and shot up about 3 feet in the air.

As the tablet disintegrated, it took longer for the pressure to build, giving her more time to hop away. Initially, my daughter was nervous about exploding rockets until she realized how harmless it really was. Now, she can’t wait to show her grandparents and cousins how to make popping rockets when we visit them for July 4th.

The Science Behind It
When water and antacid mix, carbon dioxide gas is produced. By placing the lid on the rocket, you are trapping those gas bubbles inside. As more and more bubbles are produced, the pressure inside the rocket builds, creating enough force to break the seal on the lid. There is so much force from the built up pressure that it launches the rocket into the air.
30 More Kids’ Science Experiments
Inspire kids to love science with our bundle of 30 Science Experiments !
Similar Posts

Beginning Digraph Activities

Word Family Spiders

Middle Vowel Cards and Puzzles
Pumpkin Counting Mat

Word Family Activity Pack

End of the Year Craftivity
47 comments.
- Pingback: Independence Day | Independent Activities | Bette Fetter
- Pingback: 17 Examples of STEM Project Based Learning Activities - Project Pals
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *

Build a Bubble-Powered Rocket!
Build your own rocket using paper and fizzing tablets! Watch it lift off. How high does your rocket go? Print this page for the instructions.
Suggestion:
Find a grown-up to do this activity with you.
Paper, regular 8-1/2- by 11-inch paper, such as computer printer paper or even notebook paper.
Plastic 35-mm film canister (see hints below)
Cellophane tape
Effervescing (fizzing) antacid tablet (the kind used to settle an upset stomach)
Paper towels
Eye protection (like eye glasses, sun glasses, or safety glasses)
The film canister MUST be one with a cap that fits INSIDE the rim instead of over the outside of the rim. Sometimes photography shops have extras of these and will be happy to donate some for such a worthy cause.
Keep in mind:
Just like with real rockets, the less your rocket weighs and the less air resistance (drag) it has, the higher it will go.
Making the Rocket
You must first decide how to cut your paper. You may cut it the short way or the long way to make the body of the rocket. There is no one right way to make a paper rocket. Try a long, skinny rocket or a short, fat rocket. Try a sharp nosecone or a blunt nosecone. Try it with fins or without fins. Experiment!
Here's just one idea for how you might cut your whole rocket from one piece of paper:

Cut out all the pieces for your rocket.
Wrap and tape a tube of paper around the film canister. Hint: Tape the canister to the end of the paper before you start wrapping.
Important! Place the lid end of the canister down .
Tape fins to your rocket body, if you want.
Roll the circle (with a wedge cut out) into a cone and tape it to the rocket's top.
Blasting Off
Put on your eye protection.
Turn the rocket upside down and remove the canister's lid.
Fill the canister one-third full of water.
Now work quickly on the next steps!
Drop one-half of an effervescing antacid tablet into the canister.
Snap the lid on tight.
Stand your rocket on a launch platform, such as your sidewalk or driveway.
Stand back and wait. Your rocket will blast off!
So, Dr. Marc, how does the pop-rocket work?
When the fizzy tablet is placed in water, many little bubbles of gas escape. The bubbles go up, instead of down, because they weigh less than water. When the bubbles get to the surface of the water, they break open. All that gas that has escaped from the bubbles pushes on the sides of the canister.
Now when you blow up a balloon, the air makes the balloon stretch bigger and bigger. But the little film canister doesn't stretch and all this gas has to go somewhere!
Eventually, something has to give! So the canister pops its top (which is really its bottom, since it's upside down). All the water and gas rush down and out, pushing the canister up and up, along with the rocket attached to it.
We call this wonderful and useful fact the law of action and reaction . The action is the gas rushing out of the rocket. The reaction is the rocket taking off in the other direction. In other words, for every action there is an equal and opposite reaction. The rocket goes in the opposite direction from the gas, and the faster the gas leaves the rocket, the faster the rocket gets pushed the other way.

Delta rocket similar to the one that launched the Deep Space 1 spacecraft from Cape Canaveral, Florida, in October 1998.
Real rockets work kind of the same way as the pop rocket. But instead of using tablets that fizz in water, they use rocket fuel.
The rocket that launched Deep Space 1 on October 24, 1998, had four different kinds of engines. Some pushed the rocket off the ground. Then some helped it continue its climb into space. Others gave the Deep Space 1 spacecraft its final push away from Earth. But all of them forced a gas to shoot out of the rocket, thus pushing the rocket the other way.
If you liked this, you may like:
10. What happened when you added one quarter? Two quarters?
11. Why do you think your results turned out the way they did?
12. If you could modify this experiment, what would you do differently? Be detailed in your explanation.

IMAGES
COMMENTS
Sep 6, 2022 · Students design and build paper rockets around film canisters, which serve as engines. An antacid tablet and water are put into each canister, reacting to form carbon dioxide gas, and acting as the pop rocket's propellant. With the lid snapped on, the continuous creation of gas causes pressure to build up until the lid pops off, sending the rocket into the air. The pop rockets demonstrate ...
Learn how to make a pop rocket in this quick, super fun, and easy chemical reaction science experiment. This is a new spin on the traditional Alka-Seltzer film canister pop rocket experiment. Since the old film canisters are getting harder to come by, we will use a Mini M&Ms tube to perform this chemical reaction science experiment. Materials:
Build a Pop Rocket: A Motion Experiment Introduction: Newton’s Third Law of Motion states that "for every action there is an equal and opposite reaction." This law explains what happens when two bodies exert forces on each other. Whenever an object pushes another object it gets pushed back in the opposite direction equally hard.
As more and more bubbles are produced, the pressure inside the rocket builds, creating enough force to break the seal on the lid. There is so much force from the built up pressure that it launches the rocket into the air. 30 More Kids’ Science Experiments. Inspire kids to love science with our bundle of 30 Science Experiments!
May 10, 2023 · Students build a rocket with a triangular cross section made from three rocket-shaped strips of card-stock paper and then launch it with the Pop! Rocket Launcher. Pop! Rockets [732KB PDF file] Pop! Rockets Launcher This activity is part of the Rockets Educator Guide.
Experiment with different body sizes, construction materials, and cone and fin sizes and shapes to create a different rocket. Add a payload (e.g. toy astronauts) and record what happens. Try replacing the film canister with an alternative such as mini (1 oz) playdough containers, snap-together Easter eggs or non-childproof prescription bottles ...
Fuel the Rocket 1. Ask your adult partner to help you select an appropriate area outside for the launch of your rocket. 2. Fill the canister half full of water. 3. Tape the half tablet of the effervescent antacid inside the lid of the canister using a piece of double-sided tape. 4. Close the canister, quickly place it on the launch area with ...
Dec 5, 2024 · There is no one right way to make a paper rocket. Try a long, skinny rocket or a short, fat rocket. Try a sharp nosecone or a blunt nosecone. Try it with fins or without fins. Experiment! Here's just one idea for how you might cut your whole rocket from one piece of paper: Here are the basic steps: Cut out all the pieces for your rocket.
a rocket. Be sure to tell the students to plan how they are going to use the paper. Let the students decide whether to cut the paper the short or long direction to make the body tube of the rocket. This will lead to rockets of different lengths for flight comparison. The most common mistakes in constructing the rocket are as follows:
May 13, 2021 · Activity: Controlled Propulsion Experiment - Film Canister. A film canister, Alka Seltzer, and water can demonstrate how a rocket engine works. The Alka Seltzer in the film canister, when mixed with water, produces a gas.