

7 Surface Tension Experiments To Try With Kids
- November 2, 2022
- Science Experiments
Here is a list of easy and fun surface tension experiments for kids. These surface tension experiments with water can help kids learn about static water and the forces within it.
Do you love the 4th of July milk fireworks?
What if you can create them using milk?
Have you noticed crazy little balls in your coffee mug while stirring it?
Well, it’s possible to recreate them! There are lots of other factors to know. So, let’s have a look at the seven science experiments that will help to understand physics in a better way while having fun at home.

1. Milk Fireworks
This is an easy science activity that needs only a few raw materials and can prove to be a great boredom buster.
Raw materials
- A dish
- Food coloring agents
- Liquid dish soap
Required Steps
- Take the dish and pour some milk into it.
- Now drop a few drops of coloring agents in the middle.
- Now take an earbud soaked in liquid dish soap and dip it into the middle with food coloring agents.
- The colors get scattered in all directions like fireworks!

Observation
Experiment Observing that adding little soap to the milk weakens its surface tension by pushing the milk molecules with its hydrophobic ends. Also, the food coloring agents are pushed along with them, and end up having a spectacular sight of fireworks on liquids !
Note: You can conduct this experiment with milk at different temperatures such as warm and very cold to see whether this will make any difference to the behavior of the milk molecules.
2. Water BBS
This experiment demonstrates how crazy little balls notice in the coffee mug while stirring it.

- One cup of coffee
- One coffee stirrer
- Few drops of liquid soap
Required steps
- Take the coffee mug and stir it with the stirrer
- Maybe nothing will happen, and then mix a few drops of liquid soap
In this experiment, notice some little balls in the coffee mug, which are nothing but anti-bubbles. These bubbles are formed when a liquid is dropped turbulently into the same or another liquid.
These are thin films of gas enclosing a sphere of liquid that can appear and then get fully submerged in the liquid.
Unlike ordinary air bubbles, these anti-bubbles do not rise quickly on the top. Patient to see them as they are quite mesmerising.
3. Soap Boat
This science activity video on a soap boat experiment is all about the surface tension of water and the impact of soap on water.

- 1 dish containing water
- 1 little boat with a notch out of a card
- A few cotton buds
- Liquid soap
- Take the dish and place the little paper boat on the surface of the water
- Now, soak the cotton bud in liquid soap and touch its tip into the water to power your paper boat
In this experiment, the boat will start moving swiftly! Now, this happens when you touch the soap on the surface of the water. Soap weakens its surface tension and creates enough force to push the lightweight paper boat. Interesting to notice it!
4. Floating Card

- 1 open jar with a mesh screen on its mouth
- 1 card
- 1 jug of water
- Take the open jar with the mesh screen and pour water into it from the jug
- Now, take the card and place it gently in the mouth of the jar
- Invert the jar, and you will see that it will uphold the card!
- Next, gently remove your hand from the card
- Slid out the card from beneath the jar
- The jar will hold up the water mysteriously!
Observation
Observations help to notice the mysterious water suspension. So, the science behind this floating water trick is nothing but the surface tension across the screen, which holds up the water.
There is also a role of cohesion to play in this science activity. It is the cohesion that causes surface tension. Here, water molecules remain joined together between each tiny opening of the screen mesh and form a thin invisible membrane that is strong enough to hold the water when the jar is inverted.
You can even stick some needles inside the jar! Interestingly, the surface tension will successfully prevent the water from falling in that case too!
You can use this experiment as a magic trick before your friends and can, later on, explain to them the science behind the water suspension.
5. Suddenly sinking paper clips
This science activity video on paper clip floating and sinking is again about the surface tension of water.

- 1 glass containing water
- 1 paper clip
- 1 piece of tissue paper
- A small quantity of liquid soap
- Take the paper clip and place it on top of the water surface of the glass
- Try to balance it on the water surface
- If it sinks, take it out from the glass
- Now, place the piece of small tissue paper on the water surface and then put the paper clip on it
- Next, gently remove the tissue paper from beneath the paper clip as it will start floating on the water surface
- Now take the Q-tip and soak it in liquid soap and touch its tip into the water
- The paper clip will again sink at the bottom!
Now, wondering why is the paper clip floating on water soap? Well, the reason is again the humble surface tension!
In the second step, then try to make the paper clip float on the water surface, it sinks because the metal with which the clip is made is denser than the water.
However, when placing it on a piece of floating tissue paper, it does not sink because now the surface tension of the water is supporting it.
Again, when you touch the water with soap, this surface tension gets reduced. So, the clip sinks like a brick into the glass.
Also, let’s experiment with this interesting activity with different lightweight objects to see whether the same thing is happening again.
6. Penny Dropper
Have you ever wondered how many drops of water can fit on a penny ?
Well, this super fun science activity will give all the answers.

- 1 plastic dropper
- Take the penny and place it on a flat surface
- Now take the dropper, fill it with water, and put a drop of water at the center of your penny
- Keep on adding water drops to the penny and count
- A dome shape made up of water drops will form on the penny
The experiment makes us observe that a penny can hold several water drops before it eventually starts spilling over the coin. Here, it is the surface tension of water that prevents the water molecules from falling apart. So, the water molecules remain together and form a dome shape. Even Experimenting with other liquids such as saltwater, milk, and soapy water to figure out whether they yield the same result or not.
7. Leidenfrost Effect
Have you ever heard about the Leindenfrost effect ?
Well, it is a phenomenon where liquids, instead of getting evaporated, glided on the surface of a pan. This happens when the pan is heated beyond the boiling point of that liquid.
This effect was named after the German doctor Johann Gottlob Leidenfrost (1715-1794), who described this effect.
However, to do this exciting science experiment, you will need adult supervision as this involves heat hazards!

- 1 empty pan
- One dropper
- Take the empty pan and put it on a stove.
- Next, add some water droplets into the pan one by one with the help of the dropper, and the water droplets will quickly evaporate.
- Keep on adding the water droplets but now increase your speed.
- Water droplets will now not evaporate. They will instead make small spheres gliding on the hot surface of the pan.
The observation makes us wonder how does water dance on a hot pan . See, when heating the pan more than the boiling point of water, which is 100-degree Celsius, water drops vaporize quickly that it forms a layer of steam that insulates the rest of the water droplets are added from the hot surface of the pan. As a result, you end up watching the dancing water droplets.
All the above activities can be done at home to develop a better understanding of some key concepts of physics.
Dianna Cowern, also called physics girl presented a video of seven experiments or science tricks that offer surface tension, anti-bubble, cohesion, and lienenforst effect.
Courtesy: Physics Girl

https://www.youtube.com/watch?v=WsksFbFZeeU&feature=emb_logo
https://www.antibubble.org/
https://www.stevespanglerscience.com/lab/experiments/water-screen/
https://msdlt.instructure.com/courses/108/files/2571/download?wrap=1
https://www.rookieparenting.com/how-many-drops-of-water-can-you-put-on-a-penny/
https://www.sociologygroup.com/water-drops-dance-hot-plate/
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
- Skip to primary navigation
- Skip to main content
- Skip to primary sidebar

- FREE Experiments
- Kitchen Science
- Climate Change
- Egg Experiments
- Fairy Tale Science
- Edible Science
- Human Health
- Inspirational Women
- Forces and Motion
- Science Fair Projects
- STEM Challenges
- Science Sparks Books
- Contact Science Sparks
- Science Resources for Home and School
Surface Tension of Water Demonstration
April 17, 2019 By Emma Vanstone 3 Comments
These super simple investigations are great for demonstrating the surface tension of water .
What is surface tension?
Surface tension is a force which causes a layer of liquid to behave like an elastic sheet or skin.
Molecules of water are more attracted to each other than other molecules, as water is a polar molecule. The positive hydrogen end of one molecule is attracted to the negative oxygen end of another water molecule. The surface water molecules only have air above them, so they are pulled down, creating surface tension.
The high surface tension of water allows insects to walk over it. Pond skaters have long, hairy legs, allowing them to spread their weight over a wide area. They press very gently on the water’s surface so as not to break through it.

In a container of water, molecules below the surface are pulled together ( or attracted to each other ) equally in all directions, but those on top are pulled together more tightly, as they don’t have water molecules above them; this draws them together to form a ‘skin’. It is this skin ( surface tension ) that stops items on the surface from sinking.
Surface Tension Holes Experiment
You’ll need.
A big bowl of water
Some ground pepper (black so you can see it) or any other ground product with colour

Washing up liquid ( dish soap )
Once the water settles, sprinkle the ground pepper over the top.
Drip some washing-up liqu id in the middle of the bowl and watch what happens.
A hole appears in the centre as the pepper moves outwards. This is your surface tension hole !
If you want to repeat the demonstration, you’ll need to wash out the bowl thoroughly to remove any traces of the dish soap ( washing up liquid ), or the effect won’t be as dramatic.

Why does this happen?
The surface tension hole is caused by the washing up liquid reducing the surface tension of the water. This allows the particles of water at the surface to spread out, starting from where the washing-up liquid was added.

More Surface Tension Experiments for Kids
Frugal Fun for Boys has an excellent surface tension investigation using a coin and different liquids !
You can use washing-up liquid to disrupt the surface tension of water to race lolly sticks .
In a magic milk experiment , the washing up liquid disrupts the surface tension of the milk, which makes food colouring spread out just like the pepper and water.

Another surface tension experiment is where you make a shape on the surface of the water with cocktail sticks and drop some washing-up liquid in the centre to force the sticks apart.
Watch how water behaves on the space station with this NASA video.
Try filling a bowl half full with water and carefully placing a paperclip on the top, so it floats. Mix a little washing-up liquid in a cup with water and gently pour it into the bowl; the paper clip will sink as the water can no longer support the weight of the paper clip after the washing-up liquid disrupts the surface tension of the water.
Science concepts
Surface tension

Last Updated on July 8, 2023 by Emma Vanstone
Safety Notice
Science Sparks ( Wild Sparks Enterprises Ltd ) are not liable for the actions of activity of any person who uses the information in this resource or in any of the suggested further resources. Science Sparks assume no liability with regard to injuries or damage to property that may occur as a result of using the information and carrying out the practical activities contained in this resource or in any of the suggested further resources.
These activities are designed to be carried out by children working with a parent, guardian or other appropriate adult. The adult involved is fully responsible for ensuring that the activities are carried out safely.
Reader Interactions
October 16, 2011 at 3:00 pm
Great activity, I am going to try it with my daughter! I love how you call it “washing up liquid” – I call it that too. 🙂
October 16, 2011 at 9:26 pm
Thanks, glad you like it!
October 21, 2011 at 6:01 pm
So many great ideas come form this blog! Thank you for linking up to the The Sunday Showcase
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
- Science, Tech, Math ›
Surface Tension - Definition and Experiments
Understand Surface Tension in Physics
- Physics Laws, Concepts, and Principles
- Quantum Physics
- Important Physicists
- Thermodynamics
- Cosmology & Astrophysics
- Weather & Climate
Causes of Surface Tension
Examples of surface tension, anatomy of a soap bubble, pressure inside a soap bubble, pressure in a liquid drop, contact angle, capillarity, quarters in a full glass of water, floating needle, put out candle with a soap bubble, motorized paper fish.
:max_bytes(150000):strip_icc():format(webp)/AZJFaceShot-56a72b155f9b58b7d0e783fa.jpg)
- M.S., Mathematics Education, Indiana University
- B.A., Physics, Wabash College
Surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). If the surface is between two liquids (such as water and oil), it is called "interface tension."
Various intermolecular forces, such as Van der Waals forces, draw the liquid particles together. Along the surface, the particles are pulled toward the rest of the liquid, as shown in the picture to the right.
Surface tension (denoted with the Greek variable gamma ) is defined as the ratio of the surface force F to the length d along which the force acts:
gamma = F / d
Units of Surface Tension
Surface tension is measured in SI units of N/m (newton per meter), although the more common unit is the cgs unit dyn/cm (dyne per centimeter).
In order to consider the thermodynamics of the situation, it is sometimes useful to consider it in terms of work per unit area. The SI unit, in that case, is the J/m 2 (joules per meter squared). The cgs unit is erg/cm 2 .
These forces bind the surface particles together. Though this binding is weak - it's pretty easy to break the surface of a liquid after all - it does manifest in many ways.
Drops of water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it. In the absence of gravity, the drop would minimize the surface area in order to minimize tension, which would result in a perfectly spherical shape.
Insects walking on water. Several insects are able to walk on water, such as the water strider. Their legs are formed to distribute their weight, causing the surface of the liquid to become depressed, minimizing the potential energy to create a balance of forces so that the strider can move across the surface of the water without breaking through the surface. This is similar in concept to wearing snowshoes to walk across deep snowdrifts without your feet sinking.
Needle (or paper clip) floating on water. Even though the density of these objects is greater than water, the surface tension along the depression is enough to counteract the force of gravity pulling down on the metal object. Click on the picture to the right, then click "Next," to view a force diagram of this situation or try out the Floating Needle trick for yourself.
When you blow a soap bubble, you are creating a pressurized bubble of air which is contained within a thin, elastic surface of liquid. Most liquids cannot maintain a stable surface tension to create a bubble, which is why soap is generally used in the process ... it stabilizes the surface tension through something called the Marangoni effect.
When the bubble is blown, the surface film tends to contract. This causes the pressure inside the bubble to increase. The size of the bubble stabilizes at a size where the gas inside the bubble won't contract any further, at least without popping the bubble.
In fact, there are two liquid-gas interfaces on a soap bubble - the one on the inside of the bubble and the one on the outside of the bubble. In between the two surfaces is a thin film of liquid.
The spherical shape of a soap bubble is caused by the minimization of the surface area - for a given volume, a sphere is always the form which has the least surface area.
To consider the pressure inside the soap bubble, we consider the radius R of the bubble and also the surface tension, gamma , of the liquid (soap in this case - about 25 dyn/cm).
We begin by assuming no external pressure (which is, of course, not true, but we'll take care of that in a bit). You then consider a cross-section through the center of the bubble.
Along this cross section, ignoring the very slight difference in inner and outer radius, we know the circumference will be 2 pi R . Each inner and outer surface will have a pressure of gamma along the entire length, so the total. The total force from the surface tension (from both the inner and outer film) is, therefore, 2 gamma (2 pi R ).
Inside the bubble, however, we have a pressure p which is acting over the entire cross-section pi R 2 , resulting in a total force of p ( pi R 2 ).
Since the bubble is stable, the sum of these forces must be zero so we get:
2 gamma (2 pi R ) = p ( pi R 2 ) or p = 4 gamma / R
Obviously, this was a simplified analysis where the pressure outside the bubble was 0, but this is easily expanded to obtain the difference between the interior pressure p and the exterior pressure p e :
p - p e = 4 gamma / R
Analyzing a drop of liquid, as opposed to a soap bubble , is simpler. Instead of two surfaces, there is only the exterior surface to consider, so a factor of 2 drops out of the earlier equation (remember where we doubled the surface tension to account for two surfaces?) to yield:
p - p e = 2 gamma / R
Surface tension occurs during a gas-liquid interface, but if that interface comes in contact with a solid surface - such as the walls of a container - the interface usually curves up or down near that surface. Such a concave or convex surface shape is known as a meniscus
The contact angle, theta , is determined as shown in the picture to the right.
The contact angle can be used to determine a relationship between the liquid-solid surface tension and the liquid-gas surface tension, as follows:
gamma ls = - gamma lg cos theta
- gamma ls is the liquid-solid surface tension
- gamma lg is the liquid-gas surface tension
- theta is the contact angle
One thing to consider in this equation is that in cases where the meniscus is convex (i.e. the contact angle is greater than 90 degrees), the cosine component of this equation will be negative which means that the liquid-solid surface tension will be positive.
If, on the other hand, the meniscus is concave (i.e. dips down, so the contact angle is less than 90 degrees), then the cos theta term is positive, in which case the relationship would result in a negative liquid-solid surface tension!
What this means, essentially, is that the liquid is adhering to the walls of the container and is working to maximize the area in contact with solid surface, so as to minimize the overall potential energy.
Another effect related to water in vertical tubes is the property of capillarity, in which the surface of liquid becomes elevated or depressed within the tube in relation to the surrounding liquid. This, too, is related to the contact angle observed.
If you have a liquid in a container, and place a narrow tube (or capillary ) of radius r into the container, the vertical displacement y that will take place within the capillary is given by the following equation:
y = (2 gamma lg cos theta ) / ( dgr )
- y is the vertical displacement (up if positive, down if negative)
- d is the density of the liquid
- g is the acceleration of gravity
- r is the radius of the capillary
NOTE: Once again, if theta is greater than 90 degrees (a convex meniscus), resulting in a negative liquid-solid surface tension, the liquid level will go down compared to the surrounding level, as opposed to rising in relation to it.
Capillarity manifests in many ways in the everyday world. Paper towels absorb through capillarity. When burning a candle, the melted wax rises up the wick due to capillarity. In biology, though blood is pumped throughout the body, it is this process which distributes blood in the smallest blood vessels which are called, appropriately, capillaries .
Needed materials:
- 10 to 12 Quarters
- glass full of water
Slowly, and with a steady hand, bring the quarters one at a time to the center of the glass. Place the narrow edge of the quarter in the water and let go. (This minimizes disruption to the surface, and avoids forming unnecessary waves that can cause overflow.)
As you continue with more quarters, you will be astonished how convex the water becomes on top of the glass without overflowing!
Possible Variant: Perform this experiment with identical glasses, but use different types of coins in each glass. Use the results of how many can go in to determine a ratio of the volumes of different coins.
- fork (variant 1)
- piece of tissue paper (variant 2)
- sewing needle
Place the needle on the fork, gently lowering it into the glass of water. Carefully pull the fork out, and it is possible to leave the needle floating on the surface of the water.
This trick requires a real steady hand and some practice, because you must remove the fork in such a way that portions of the needle do not get wet ... or the needle will sink. You can rub the needle between your fingers beforehand to "oil" it increase your success chances.
Variant 2 Trick
Place the sewing needle on a small piece of tissue paper (large enough to hold the needle). The needle is placed on the tissue paper. The tissue paper will become soaked with water and sink to the bottom of the glass, leaving the needle floating on the surface.
- lit candle ( NOTE: Do not play with matches without parental approval and supervision!)
- detergent or soap-bubble solution
Place your thumb over the small end of the funnel. Carefully bring it toward the candle. Remove your thumb, and the surface tension of the soap bubble will cause it to contract, forcing air out through the funnel. The air forced out by the bubble should be enough to put out the candle.
For a somewhat related experiment, see the Rocket Balloon.
- piece of paper
- vegetable oil or liquid dishwasher detergent
- a large bowl or loaf cake pan full of water
Once you have your Paper Fish pattern cut out, place it on the water container so it floats on the surface. Put a drop of the oil or detergent in the hole in the middle of the fish.
The detergent or oil will cause the surface tension in that hole to drop. This will cause the fish to propel forward, leaving a trail of the oil as it moves across the water, not stopping until the oil has lowered the surface tension of the entire bowl.
The table below demonstrates values of surface tension obtained for different liquids at various temperatures.
Experimental Surface Tension Values
Edited by Anne Marie Helmenstine, Ph.D.
- Understanding Time Dilation Effects in Physics
- 6 Kinds of Simple Machines
- What Is Mass?
- Life of Léon Foucault, Physicist Who Measured the Speed of Light
- Multiverse Definition and Theory
- What Is the Shear Modulus?
- Huygens' Principle of Diffraction
- The Difference Between Terminal Velocity and Free Fall
- What Is Time? A Simple Explanation
- What Is Young's Modulus?
- Understanding What Fluid Dynamics is
- What Is Blackbody Radiation?
- Black Holes and Hawking Radiation
- Quantum Physics Overview
- What Is the Twin Paradox? Real Time Travel
- Heinrich Hertz, Scientist Who Proved Existence of Electromagnetic Waves

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
- Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks
Surface Tension
Surface tension is a phenomenon that occurs due to the cohesive forces of liquid molecules. The molecules on the surface of a liquid are attracted by their neighbors from the sides and bottom. As a result, the liquid surface forms a “skin” with minimum surface area and energy.
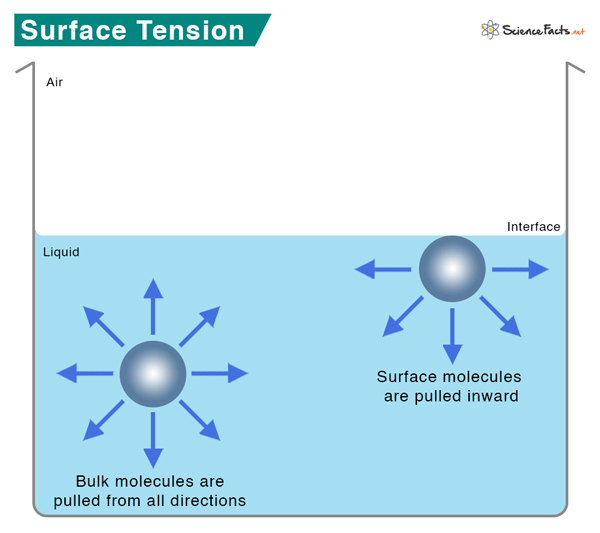
What Causes Surface Tension
Intermolecular forces like hydrogen bonding and Van der Waals forces hold the molecules together. Within the body of a liquid, molecules do not experience any force because the interactive forces from the neighboring molecules cancel out. On the surface, the molecules are attracted from the sides and bottom. No water molecules exist on the top; hence, no attractive forces from the top. As a result, the surface molecules contract and resist stretching and rupture. The surface is under tension, which explains the origin of the word surface tension.
Since surface tension refers to the interactive force, it is quantified as the force per unit length or energy per unit area. Therefore, its SI unit is Newton per meter of N/m. Its cgs unit is dyne/cm. In terms of energy units, they are Joules per square meter (J/m 2 ) in the SI system and ergs/cm 2 in the cgs system.
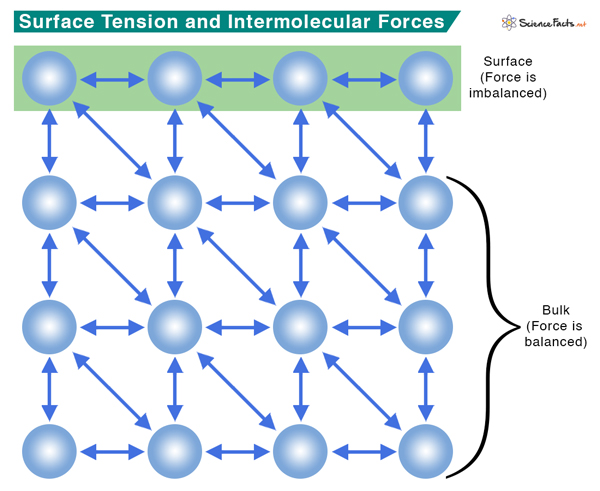
Surface Tension of Water
Water has high surface tension, which can be explained by its polarity and hydrogen bonding. Water consists of one oxygen atom flanked by two hydrogen atoms. The shape of the molecule and the electronegativity difference between hydrogen and oxygen make water a polar molecule. As a result, molecules are held together by strong hydrogen bonding. Energy is required to break these intermolecular bonds. Since water has a high surface tension, much energy is required to separate its molecules. The higher a liquid’s polarity, the higher the surface tension.
Cohesive and Adhesive Forces
Surface tension can explain cohesive and adhesive forces. Cohesive forces hold the liquid body with minimum surface area. Adhesive forces are responsible for the body to spread out. The liquid will hold its shape if the cohesive forces exceed adhesive forces. An example is mercury, whose surface tension at 25 °C is 485 mN/m. Suppose the cohesive forces are less than the adhesive forces. In that case, the liquid will spread out, thus, maximizing its surface area. This process is called wetting. Water has a surface tension of 72 mN/m and can easily wet surfaces.
The shape of water and mercury meniscuses in a test tube depends on cohesive and adhesive forces. The meniscus of water is upward or concave because water wets the surface and creeps up the side. On the other hand, the meniscus of mercury is downward or concave because mercury does not wet glass. The cohesive force is strong enough to form a drop.
Examples of Surface Tension
1. Objects floating on water – Objects can float on the water’s surface if they do not rupture the “skin” and separate the molecules. The surface behaves like an elastic membrane that can resist the object’s weight. Small insects like water striders can walk on water because their lightweight bodies cannot penetrate the surface. It shows that surface tension is important to life. The surface tension of water can also support needles and razor blades.
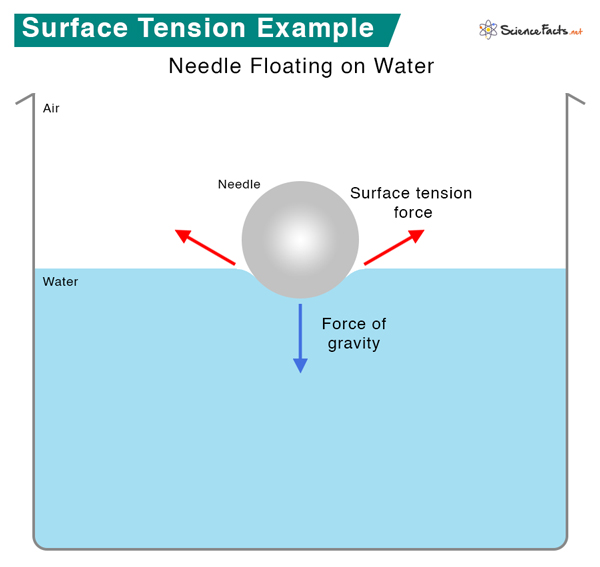
2. Jaundice test – Powdered sulfur is spread over urine. It will remain on the surface if urine is free of bile. However, bile reduces the surface tension of urine. If bile is present in urine, the sulfur powder will sink.
3. Hot water, soaps, and detergents – Hot water has a lower surface tension than cold water. Therefore, it is an excellent “wetting” agent. Soaps and detergents further reduce surface tension so water can enter pores and soiled parts. Therefore, detergents added to hot water make them perfect for washing clothes.
4. Liquid droplets – They are round because the cohesive forces of the surface layer pull into a spherical shape. For this reason, surface tension is an important property to study.
5. Surface tension disinfectants – Disinfectants are usually solutions of low surface tension. Therefore, they can spread out on the cell walls of bacteria and disrupt them.
6. Tents – Tents used for camping are made of materials that prevent rainwater from penetrating inside. The surface tension of water bridges the pores in the finely woven material. However, touching the tent will break the surface tension, seeping water through the tent.
Surface Tension Values
The following table gives the surface tension values of different liquids at 20 °C.
Ans. Increasing the temperature increases the kinetic energy of the molecules. As the molecules get agitated, they lose the efficiency of intermolecular attraction. Due to reduced cohesive forces, the surface tension decreases as temperature increases.
Ans. No, surfactants are designed to reduce surface tension. They allow liquid to wet surfaces.
Ans. The presence of soluble particles in water may increase or decrease surface tension. The surface tension will decrease if the impurities are less soluble (for example, camphor). On the other hand, the surface tension will increase if the impurities are more soluble (for example, salt).
- Surface Tension and Water – Usgs.gov
- Surface Tension – Hyperphysics.phy-astr.gsu.edu
- Surface Tension – Dataphysics-instruments.com
- Surface Tension – Chem.libretexts.org
- Surface Tension: Definition and Experiments – Thoughtco.com
- Surface Tension – Chem.purdue.edu
Article was last reviewed on Friday, October 6, 2023
Related articles

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.

IMAGES
COMMENTS
Now, this happens when you touch the soap on the surface of the water. Soap weakens its surface tension and creates enough force to push the lightweight paper boat. Interesting to notice it! 4. Floating Card. This science activity video on a soap boat experiment is all about the surface tension of water and the impact of soap on water.
Magic milk experiment to demonstrate surface tension. Another surface tension experiment is where you make a shape on the surface of the water with cocktail sticks and drop some washing-up liquid in the centre to force the sticks apart. Watch how water behaves on the space station with this NASA video.
Examples of Surface Tension . Drops of water. When using a water dropper, the water does not flow in a continuous stream, but rather in a series of drops. The shape of the drops is caused by the surface tension of the water. The only reason the drop of water isn't completely spherical is that the force of gravity pulling down on it.
Surface tension is a fascinating phenomenon that is often overlooked. It is the force that allows insects to walk on water, and it is also responsible for the formation of bubbles. In this article, you will learn about the science behind the interesting force and how to perform simple surface tension experiments to demonstrate its effects.. Surface tension is a powerful force that can have ...
Surface tension holds the surface molecules of liquids tightly together and makes for some fun experiments!
Surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Since these intermolecular forces vary depending on the nature of the liquid (e.g. water vs. gasoline) or solutes in the liquid (e.g. surfactants like detergent), each solution exhibits differing surface tension properties.
You've seen examples of surface tension in action: water striders walking on water, soap bubbles, or perhaps water creeping up inside a thin tube. What, exactly, is surface tension? ... In this experiment, you will be making and using a single beam balance to measure the force exerted by surface tension on a needle, floating on the surface of ...
An example is mercury, whose surface tension at 25 °C is 485 mN/m. Suppose the cohesive forces are less than the adhesive forces. In that case, the liquid will spread out, thus, maximizing its surface area. ... Surface Tension: Definition and Experiments - Thoughtco.com; Surface Tension - Chem.purdue.edu; Article was last reviewed on ...
This short (<5-10 minutes) pair of demonstrations uses glass slides with a very thin film of water to demonstrate the cohesive and adhesive forces of water molecules, and a needle floating on water to demonstrate surface tension.
Experiment with surface tension and temperature changes to show upwards, downwards and sideways particle movement. ... the liqueur variation starts with a sideways movement and is an example of solutal convection. This curious effect arises from a combination of several factors associated with particles: diffusion, surface tension and density.